Significance Statement
Real-time dynamics of renal autoregulation to stabilize renal blood flow and GFR and protect glomeruli from hypertension in conscious animals with spontaneously fluctuating BP remain uncharacterized. Using novel methods to analyze relationships between BP and renal blood flow, the authors show that conscious rats display autoregulatory restoration of renal blood flow in <10 seconds; restoration is significantly slower with impaired autoregulation, resulting in exposure to increased glomerular pressure. However, unlike rats under anesthesia, conscious rats achieve complete compensation, even with severe autoregulatory impairment after calcium channel blockade. These data indicate that transient glomerular pressure elevations may play a larger role in hypertensive glomerulosclerosis than recognized, and suggest that mechanisms independent of voltage-gated calcium channels might maintain overall renal blood flow and GFR stability when known autoregulatory mechanisms are impaired.
Keywords: hypertension, renal hemodynamics, nephrosclerosis, glomerulosclerosis
Abstract
Background
Renal autoregulation maintains stable renal function despite BP fluctuations and protects glomerular capillaries from hypertensive injury. However, real-time dynamics of renal autoregulation in conscious animals have not been characterized.
Methods
To develop novel analytic methods for assessing renal autoregulation, we recorded concurrent BP and renal blood flow in conscious rats, comparing animals with renal autoregulation that was intact versus impaired (from 3/4 nephrectomy), before and after additional impairment (from the calcium channel blocker amlodipine). We calculated autoregulatory indices for adjacent short segments of increasing length (0.5, 1, 2.5, 5, 10, and 20 seconds) that exhibited a mean BP difference of at least 5 mm Hg.
Results
Autoregulatory restoration of renal blood flow to baseline after BP changes in conscious rats occurs rapidly, in 5–10 seconds. The response is significantly slower in states of impaired renal autoregulation, enhancing glomerular pressure exposure. However, in rats with severe renal autoregulation impairment (3/4 nephrectomy plus amlodipine), renal blood flow in conscious animals (but not anesthetized animals) was still restored to baseline, but took longer (15–20 seconds). Consequently, the ability to maintain overall renal blood flow stability is not compromised in conscious rats with impaired renal autoregulation.
Conclusions
These novel findings show the feasibility of renal autoregulation assessment in conscious animals with spontaneous BP fluctuations and indicate that transient increases in glomerular pressure may play a greater role in the pathogenesis of hypertensive glomerulosclerosis than previously thought. These data also show that unidentified mechanosensitive mechanisms independent of known renal autoregulation mechanisms and voltage-gated calcium channels can maintain overall renal blood flow and GFR stability despite severely impaired renal autoregulation.
Hypertension (HTN) plays a major role in the progression of most CKDs, including diabetic nephropathy.1–6 Indeed, despite attempts to identify additional therapeutic targets, aggressive BP control and renin-angiotensin system blockade remain the mainstays of current CKD management.5–9 And even the beneficial effects of renin-angiotensin system blockade are at least partly dependent on BP lowering.5,6,9,10 Considerable evidence also indicates that hypertensive glomerular damage depends on the degree to which HTN is transmitted to the renal microvasculature rather than its severity per se.5,6,9–11 Renal autoregulation (AR) represents the primary mechanism for preventing glomerular transmission of BP elevations, episodic or sustained, and for maintaining the essential stability of renal blood flow (RBF), GFR, and glomerular pressure (PGC) despite such fluctuations.5,6,9,12–20 When renal AR is intact, as it is in most patients with essential HTN, and BP remains within the AR range, glomeruli are protected, and only the slowly progressive vascular pathology of benign nephrosclerosis is observed.5,9,16,19,21 The importance of this protection is illustrated by the very small absolute individual risk of ESKD in essential HTN (<0.5%).5,6,9 However, if severe HTN exceeds the autoregulatory range, malignant nephrosclerosis develops.5,9,19,21–23 The spontaneously hypertensive rat and its stroke-prone counterpart provide experimental illustrations.9,10,16,19–21,24–27 Both have intact AR, but after salt supplementation only the spontaneously hypertensive stroke-prone rats develops HTN that is severe enough to result in malignant nephrosclerosis. Conversely, when renal AR is impaired as in renal mass reduction (RMR) models of CKD, the glomerular transmission of even modest HTN greatly reduces the BP threshold at which hypertensive glomerulosclerosis starts to develop, as also observed in clinical CKD.5,6,9,10,19–21,28–30 Moreover, additional AR impairment produced by superimposed calcium channel blockers (CCBs) in RMR models predictably increases the severity of glomerulosclerosis at any given BP.5,6,9,10,19,31
However, to date such differences in AR capacity have been demonstrated only under anesthesia. Furthermore, the autoregulatory index used to assess AR capacity is calculated solely on the basis of the magnitude of the steady-state autoregulatory compensation achieved, i.e., the degree to which RBF is restored to baseline after a step-BP change.12–14,16,17,20,24,30–33 Although such assessments have provided important qualitative insights, they have significant limitations. AR is not instantaneous and BP does not change from one steady-state to another but rather fluctuates continuously in the conscious state.16–20,29–31,34–36 Accordingly, step-AR responses under anesthesia cannot reflect what must be a very dynamic process in the conscious state with both the magnitude and the rate of the AR response determining how much of a given BP fluctuation is transmitted to the glomeruli and for how long. Moreover, such AR responses in the conscious state are also likely to be modulated by other concurrent and time-varying non-BP neurohumoral inputs to the renal vasculature. Thus, little is known about the real-time dynamics of renal AR and PGC transmission occurring with spontaneous BP fluctuations in the conscious state. The objective of these studies was to develop novel analysis methods that would provide insights into the real-time mechanics of renal AR and glomerular BP transmission in the conscious state by comprehensively interrogating the simultaneously obtained BP and RBF data from conscious rats with both intact (preserved step-AR) and reduced (>75%) renal mass (impaired step-AR). Given that CCBs impair AR in normal rats and dogs16,17,20,37–39 and essentially abolish the already impaired AR capacity in RMR models,5,6,9,10,16,31 the effects of superimposed administration of the dihydropyridine CCB amlodipine on the dynamics of pressure-flow relationships were additionally examined.
Methods
Experimental Design and Methods
Animals
Studies were performed on male Sprague–Dawley rats (Charles River Laboratories) weighing 250–350 g, fed a standard 1% NaCl rat chow, and provided water ad libitum. All animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals and the protocols approved by the Hines Veterans Affairs Institutional Animal Care and Use Committee.
Surgical Procedures and Experimental Design
Investigations were performed in rats that underwent either sham surgery (intact renal mass; normal controls) or approximately 3/4 RMR by surgical excision (RK-NX), and were chronically instrumented with a BP radiotransmitter (Data Sciences, Inc.) as previously described.30,40 After 2–3 weeks of recovery and compensatory adaptations, a RBF transducer (model 1RB; Transonic Systems, Ithaca, NY) was placed on the left renal artery of all rats as previously described.26,30,39,41,42 After another week of additional recovery, BP and RBF recordings were obtained at a sampling rate of 200 Hz for 1–4 hours on one to three separate occasions at 24-hour intervals in conscious rats. After these baseline BP and RBF measurements, both sets of rats received amlodipine in drinking water (100 mg/L).39 However, because of an approximately 50% greater fluid intake on average in RK-NX as compared with control rats,43 the amount of amlodipine received by the RK-NX rats was likely to have been on average 50% greater. After approximately 48–72 hours of amlodipine, two to four BP and RBF recordings were repeated on separate days (Table 1). Recordings were obtained between 9:00 am and 3:00 pm. The level of sleep/activity in individual rats was observed to vary during the recordings but was not specifically monitored. The following methodologies were used to analyze the BP–RBF relationships.
Table 1.
Number of segments used for SSARI calculations for BP change event of at least 5 mm Hg between adjacent segments and the mean BP change for each segment length
| Group | Segment Length | |||||
|---|---|---|---|---|---|---|
| 0.5 s | 1 s | 2.5 s | 5.0 s | 10 s | 20 s | |
| Intact (n=10) | ||||||
| No. of segments | 993±156 | 668±98 | 321±44 | 165±24 | 61±8 | 27±4 |
| BP change, mm Hg | 6.7±0.06 | 7.2±0.07 | 7.6±0.15 | 7.6±0.19 | 7.3±0.20 | 7.4±0.20 |
| Intact plus amlodipine (n=10) | ||||||
| No. of segments | 1229±164 | 862±106 | 452±43 | 239±25 | 98±11 | 48±6 |
| BP change, mm Hg | 6.7±0.07 | 7.3±0.06 | 7.6±0.08 | 7.8±0.14 | 7.6±0.16 | 7.5±0.20 |
| RK-NX (n=15) | ||||||
| No. of segments | 7364±989 | 4841±655 | 2532±325 | 1151±147 | 360±45 | 136±20 |
| BP change, mm Hg | 7.5±0.22 | 8.2±0.25 | 8.9±0.31 | 8.5±0.21 | 7.4±0.11 | 7.1±0.16 |
| RK-NX plus amlodipine (n=15) | ||||||
| No. of segments | 2076±372 | 1179±213 | 555±107 | 261±52 | 105±22 | 47±12 |
| BP change, mm Hg | 7.2±0.19 | 7.7±0.19 | 7.6±0.15 | 7.3±0.10 | 6.8±0.11 | 6.7±0.16 |
Data are shown as mean±SEM.
Assessment of AR Efficiency through a Novel Analysis Methodology using Calculation of Short-Segment Autoregulation Indices in Conscious Rats
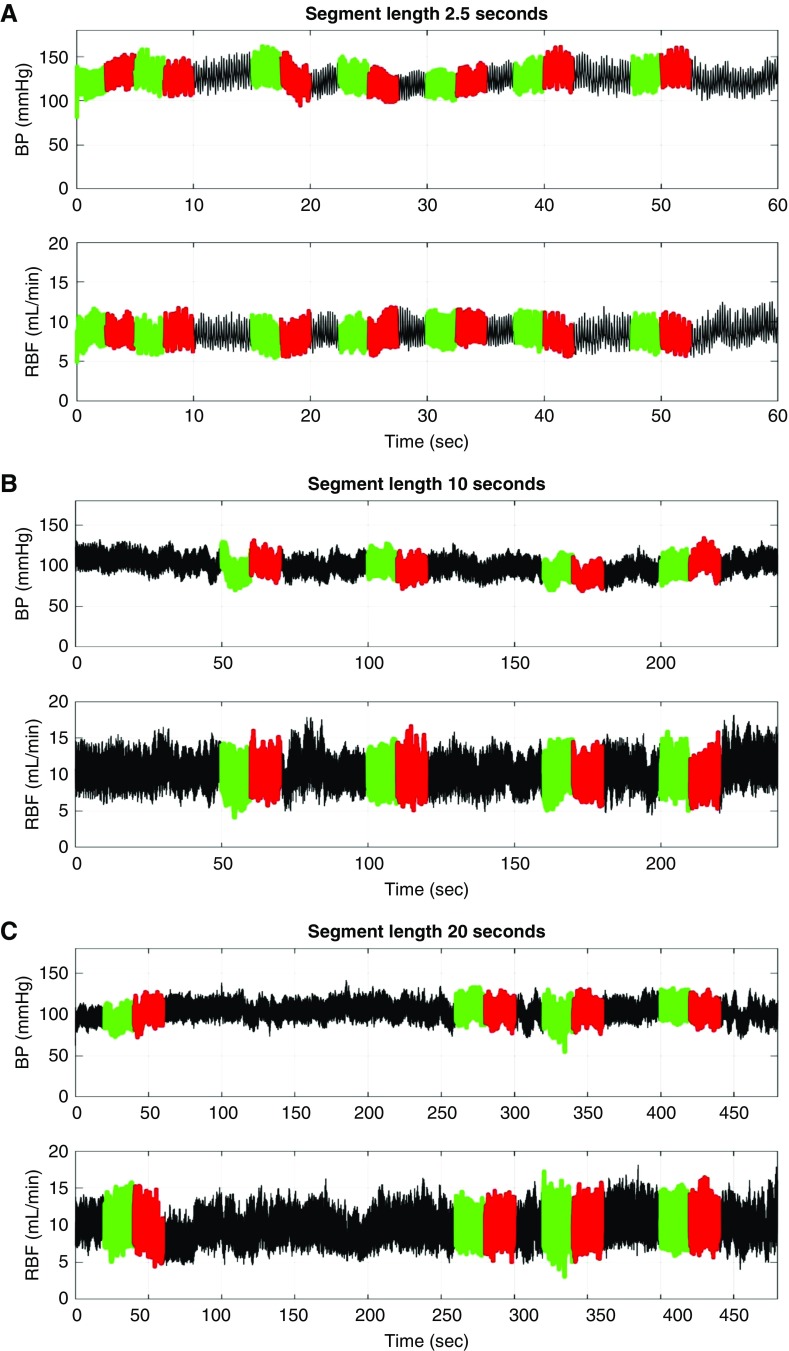
Each BP and RBF recording (1–4 hours each) was low-pass filtered (cut-off 10 Hz), down-sampled (20 Hz), and divided into short segments of increasing length (0.5, 1, 2.5, 5, 10, and 20 seconds). Then adjacent short segments for each segment length that exhibited a mean arterial pressure (MAP) difference of at least 5 mm Hg were identified as illustrated in Figure 1, A–C and Supplemental Figure 1, A and B. It was reasoned that an increasing duration of exposure to a BP change (length of the following adjacent segment with a BP difference) would permit greater time for AR compensation and thereby allow the time course of the AR response to be inferred. It was recognized that with increasing segment length, the potential for BP variations within the segment as well as for the modulation of AR responses to BP events by non-BP neurohormonal vascular signals would also increase. But, it was thought that despite such limitations, the composite responses as assessed by the short-segment autoregulatory index (SSARI) methodology would nevertheless provide insights relevant to BP transmission in the conscious state. Accordingly, AR indices were calculated for all such available adjacent segment pairs, using standard formulae (fractional change in RBF/fractional changes in MAP), with an SSARI of 0.0 indicating perfect autoregulatory compensation and a value of approximately 1.0 indicating an absence of autoregulatory compensation. For each segment length, the average percentage of segments available for such SSARI calculations ranged between 4% and 21%, and the absolute number between approximately 20 and approximately 9000, depending upon the length of the segment and the number and length of the available BP and RBF recordings (Table 1). The results for each segment length for all recordings for an individual rat were averaged and then separated into appropriate groups for group comparisons. The very infrequently occurring SSARI that exceed 3.1 in magnitude were excluded from the averages as they were considered to represent effects of noise or other anomalies in the data. Table 1 also provides the data for the mean BP change associated with the segment pairs of each segment length that were utilized for SSARI calculations.
Figure 1.
Illustration of the methodology for SSARI analysis used for simultaneous BP and RBF recordings obtained in conscious rats. Adjacent segments of increasing length (0.5, 1, 5, 10, and 20 seconds) that exhibited a MAP difference of at least 5 mm Hg were identified. Autoregulatory indices are calculated for each adjacent segment pair (fractional change in RBF/fractional change in MAP) of each of the segment lengths. Illustrated are such adjacent segments with lengths: (A) 2.5 seconds, (B) 10 seconds, and (C) 20 seconds. The number of segments used for each segment length for SSARI calculations are provided in Table 1.
Analyses were also performed to examine if the calculated SSARI differed with BP increases versus BP decreases. The effect of increasing the threshold of BP change required between adjacent segments before the calculation of SSARI to at least 10 mm Hg rather than 5 mm Hg was also assessed. The number of segments available and the BP change associated with the 10 mm Hg threshold for adjacent segments of each segment length are also provided (Supplemental Material, Supplemental Table 1). Additional SSARI analyses were performed for segments that were selected for minimal BP variation (<2.5 mm Hg) during the 10 seconds after and 5 seconds before the BP change to more closely mimic the AR response during step-AR studies under anesthesia. The number of segments available for such analysis were considerably smaller and on average, ranged between 10 and 40 per rat. Very few segments longer than 10 seconds that fulfilled this BP stability criteria were available for analysis.
Comparison of SSARI Analysis in the Conscious versus Anesthetized State in the Same Rats
These studies were undertaken because of the large differences that were observed between the renal AR responses in conscious rats inferred through the SSARI methodology versus the classic step-AR responses observed under anesthesia, in states of impaired renal AR. The objective was to compare SSARI patterns in conscious rats with preserved AR (control rats with intact renal mass) and those with impaired AR (RK-NX rats with and without amlodipine) to the SSARI patterns observed under anesthesia in the same rats. Accordingly, simultaneous BP and RBF recordings were obtained in additional control rats, RK-NX rats, and RK-NX rats receiving amlodipine for 2–4 days, after which the rats were anesthetized (isoflurane). Because of a lack of spontaneous BP fluctuations under anesthesia, SSARI analysis could only be performed after a step-BP increase was produced mechanically using vascular miniclamps, as previously described by us and others.26,28,30–32,39,40,43–46 One to three such steps were used in each rat. The step increases in MAP (mean±SEM) for intact control, RK-NX, and RK-NX plus amlodipine rats averaged 27.1±4.9, 29.9±3.1 and 14.3±2.0 mm Hg, respectively. After the step-BP increases, RBF was followed for and allowed to stabilize over at least 2–3 minutes as illustrated in Supplemental Figure 1C. The SSARI values were computed for various segment lengths by taking segments just before and just after each of these points of step-BP increase. Accordingly, one to three segments of each length were available for SSARI calculations in each rat. To facilitate comparison with SSARI values obtained during step-AR studies under anesthesia, SSARI values were additionally computed for segment lengths of 60 and 120 seconds for the conscious recordings despite the potential limitations associated with the use of longer segments (see above).
Conventional Transfer Function Methodology for Assessment of “Dynamic AR” in Conscious Rats
This is the methodology that has most often been used to assess dynamic AR function, including in conscious animals.17,20,26,30,32–36,39 Although we have previously shown that this methodology does not yield consistent estimates of AR compensation/impairment in states of impaired AR,30,39 it does provide evidence of alterations in operational characteristics of the myogenic and TGF mechanisms of AR in such states.16,17,20,30,39 Therefore, the primary objective of these studies was to confirm the presence of such alterations in transfer function that have been described after RMR and CCBs in this set of rats undergoing the SSARI studies. Contiguous subsegments of 30 minutes duration from each recording that were free of noise or other artifacts were resampled at 20 Hz using a 10 Hz low-pass antialiasing filter as previously described.26,30,39,41 The BP and RBF power spectra were determined using the Welch averaged periodogram method after linear trend removal. Input and output autopower spectra and crosspower spectra were calculated for each segment, averaged, and the admittance functions, coherence, and fractional gain in admittance (FGA) were calculated as previously described.26,30,39,41 Comparisons could only validly be performed for those rats in whom such 30 minute contiguous segments without noise or artifacts were available both at baseline and after amlodipine administration. (Of note, such a restriction is not applicable to the SSARI analysis, which can be conducted on any noise- and artifact-free section of the data that is twice the segment length of interest i.e., 1–40 seconds, given the individual segment lengths of 0.5–20 seconds.)
The “Bin Analysis” Methodology to Assess Overall RBF Stability during Spontaneous BP Changes
We have recently described the use of this methodology to detect BP related effects on RBF stability during chronic infusions of angiotensin II, phenylephrine, and N(ω)-nitro-L-arginine methyl ester in conscious rats.41,42 After resampling to 20 Hz as described above, all available BP and RBF recordings were divided into 1, 2.5, 5, 10, 30, 60, and 100 second segments that are free of noise and artifact, with 50% overlap between successive segments as previously described.41,42 For each segment length, the BP values are averaged for individual segments and placed into BP bins of 10 mm Hg ranging from 80 to 140 mm Hg, whereas the RBF values for each segment were associated to the corresponding BP bin. The RBF–BP bin data were averaged over the multiple recordings at baseline and after amlodipine for each rat. The RBF–BP bin data were then averaged across rats. The RBF values for each rat were expressed as the percentage of the respective RBF at their average baseline BP. Only the bins for which data were available for >50% of the rats and with at least 5% of the BP values in the bin were used for analysis.
Statistical Analyses
Nonparametric univariable statistical analyses for continuous outcomes with small sample sizes were used to compare the SSARI results for the four groups of data sets at each time point (t=0.5, 1, 2.5, 5, 10, and 20 seconds). Medians and interquartile ranges are used to summarize distributions of the variables within each group. Wilcoxon rank-sum tests were used to make pairwise comparisons between independent groups (intact versus RK-NX), whereas Wilcoxon signed-rank tests were used for comparisons of groups consisting of the same rats (e.g., intact versus intact plus amlodipine, RK-NX versus RK-NX plus amlodipine). Dunn–Sidak corrections were used to account for the inflation of type 1 error for multiple comparisons at different time points. Specifically, the significance level was adjusted from α=0.05 to α*=0.0127–0.006, depending upon the number of comparisons. All P values were compared with α* when assessing significance as indicated in the respective tables and figure legends. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
The Novel SSARI Methodology Allows Demonstration of Significant Differences in AR Responses between Conscious Rats with Intact and Reduced Renal Mass
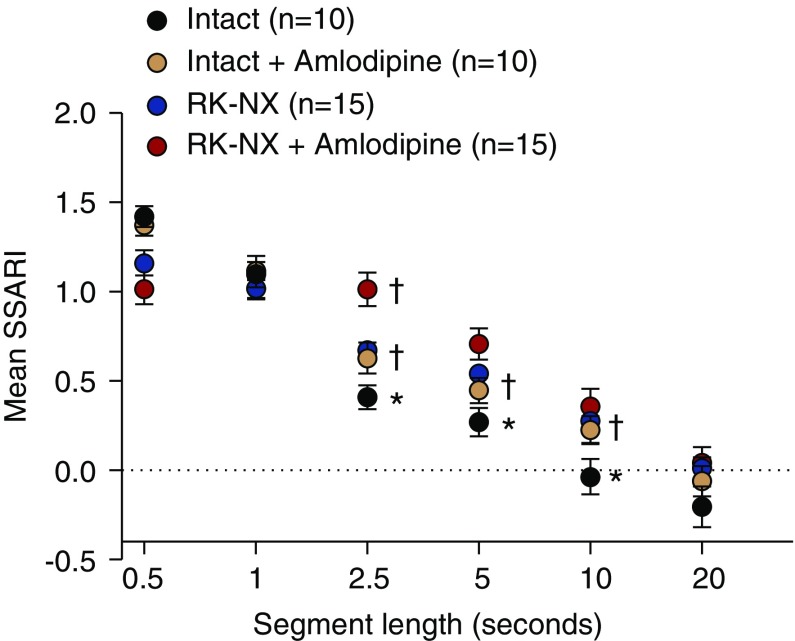
The BP and RBF averages for conscious intact and RK-NX rats before and after amlodipine are presented in Table 2 and are similar to those reported previously from our laboratory.30,39,41 As noted in the Methods section, the hemodynamic responses associated with BP change events are analyzed from an autoregulatory perspective by the SSARI methodology. Figure 2 demonstrates the effect of segment length on the calculated SSARI in intact and RK-NX rats. Of note, with a segment length of 0.5 seconds, an SSARI of >1.0 is observed in control rats with intact renal mass indicating that the fractional change in RBF exceeds the fractional BP change in the same direction. This initial response is identical to that observed after a step-BP change is imposed under anesthesia and is attributed to an initial transient passive change in vascular compliance.20,32,33,46 Perhaps not surprisingly, this is attenuated in RK-NX rats with preexistent vasodilation.30,40 Compliance returns to baseline by approximately 1.0 second as indicated by the fractional change in BP and RBF becoming equal in both control and RK-NX rats (SSARI of approximately 1.0 with segment length of 1.0 seconds). The subsequent initiation of the active autoregulatory compensation in response to the preceding BP change results in a progressive decline in SSARI with increasing segment length. However, with 2.5-second segments, RK-NX rats exhibit significantly less AR compensation than controls. With further increases in segment lengths to 5 and 10 seconds, the SSARI for both intact and RK-NX rats show a progressive decline indicating better time-dependent AR compensation, although the differences between the two groups persist. Complete compensation (SSARI of approximately 0.0) is attained in control rats with segment length of 10 seconds versus 20 second segments for RK-NX rats. Thus, SSARI calculated from 2.5-second segments provide the clearest discrimination between rats with preserved versus impaired AR. Similarly, the AR impairment (increases in SSARI) by amlodipine in both control and RK-NX rats is most clearly demonstrated with 2.5-second segments. But even in these CCB-treated rats, AR compensation improves with increasing segment length, and complete compensation is achieved with segment lengths of 20 seconds (SSARI approximately 0). The underlying autoregulatory nature of the hemodynamic responses to BP changes in conscious rats is supported by the very similar SSARI results with increases versus decreases in BP, with a 10 mm Hg versus 5 mm Hg BP change threshold (Supplemental Figure 2), or when the SSARI analysis is restricted to a much smaller number of 10-second segments selected for minimal BP variation after a qualifying BP event (Supplemental Figure 3).
Table 2.
Hemodynamic data in conscious intact and RK-NX rats before and after amlodipine
| Group (n) | Average No. of Recordings per Rat | MAP, mm Hg | Left RBF, ml/min | Conductance, ml/min per mm Hg |
|---|---|---|---|---|
| Intact (n=10) | 1.9±0.15 | 102.6±2.2 | 7.00±0.5 | 0.07±0.01 |
| Intact plus amlodipine (n=10) | 2.8±0.25 | 94.2±2.2a | 7.6±3.3 | 0.08±0.01 |
| RK-NX (n=15) | 3.7±0.19 | 126.4±3.2b | 12.9±1.5b | 0.1±0.01 |
| RK-NX plus amlodipine (n=15) | 2.4±0.27 | 105.8±2.0a | 13.6±1.8 | 0.13±0.02a |
Results are shown as mean±SEM.
P<0.01 maximum after amlodipine versus respective intact or RK-NX at baseline; P values >0.01 were considered nonsignificant (see statistical analysis methods for explanation).
P<0.01 maximum versus intact.
Figure 2.
Demonstration of improving autoregulatory compensation with increasing segment length as indicated by the calculated SSARI (mean±SEM) in rats with intact and RK-NX. The effects of the CCB amlodipine on this relationship in both intact control and RK-NX rats are additionally illustrated. With a segment length of 0.5 seconds, the calculated SSARI exceeded or tended to exceed 1.0 (in all groups) indicative of the initial passive vascular responses to BP change before the onset of AR. With 1-second segments, SSARI in all groups averaged approximately 1.0, indicating an absence of autoregulatory response within this time frame. However, with 2.5-, 5-, and 10-second segments, significant differences were observed between intact and RK-NX rats in the extent of autoregulatory compensation achieved (1-SSARI) (*P<0.009 maximum). In both intact and RK-NX groups, progressive declines in SSARI were observed, with longer segment lengths indicating better time-dependent autoregulatory compensation. However, consistent with the slower autoregulatory responses in RK-NX rats, complete compensation (SSARI of approximately 0.0) was observed with a segment length of 10 seconds in intact rats, but such was only seen with 20-second segments in RK-NX rats. The adverse effects of amlodipine on autoregulatory responses in both intact and RK-NX rats are likewise most clearly observable with segment lengths of 2.5 seconds (†P<0.007 maximum versus respective intact and RK-NX rats without amlodipine). As in RK-NX rats, an SSARI of approximately 0.0 was not observed with segment lengths of <20 seconds in rats that had received amlodipine. The adjusted significance level on the basis of the Dunn–Sidak correction for multiple comparisons was P<0.009.
The SSARI Methodology Demonstrates a Gaussian Distribution Pattern of AR Responses in Conscious Rats with Both Preserved and Impaired AR
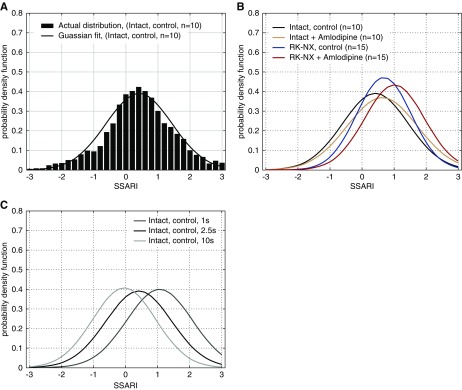
Figure 3A shows a Gaussian distribution of the wide range of individual SSARI values for 2.5-second segments in control intact rats (similar median and mean values as observed for most biologic variables). The SSARI distribution profile for RK-NX rats for 2.5-second segments exhibits a similar Gaussian distribution, but with a rightward shift consistent with the overall reduced AR efficiency (Figure 3B). Administration of amlodipine likewise produced a rightward shift in the SSARI distribution profiles in both intact control and RK-NX rats. The effect of segment length on the SSARI distribution profile in intact control rats is illustrated in Figure 3C. Although changes in segment length clearly shift the SSARI distribution profiles, they continue to exhibit a roughly Gaussian pattern and therefore for ease of visualization are depicted by their Gaussian approximants.
Figure 3.
Autoregulatory responses (SSARI) exhibit a normal Gaussian distribution. (A) Intact rats, 2.5 second segments; (B) SSARI distribution profiles (2.5-second segments) in intact versus RK-NX rats before and after amlodipine, illustrating the shift to the right with autoregulatory impairment; (C) illustration of the better autoregulatory compensation observed with an increasing segment length (time) in intact rats. Of note, the variances remain relatively unchanged.
The SSARI Methodology Demonstrates that the Compensatory Efficiency of the AR Response Is Modulated by the Ambient BP Levels
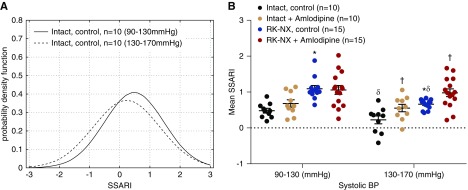
Figure 4A shows that the SSARI distribution profile for 2.5-second segments in intact control rats represented by its Gaussian approximants is shifted to the left (greater AR compensation) when the ambient mean BP is higher (130–170 mm Hg versus 90–130 mm Hg). Figure 4B presents the mean SSARI values for 2.5-second segments for these ambient BP ranges for both intact and RK-NX rats before and after amlodipine. Higher ambient BP significantly improved the AR compensation in both intact and RK-NX rats, but the significant differences between the two groups persisted. However, amlodipine abolished this effect of ambient BP on SSARI.
Figure 4.
Higher ambient pressures are associated with stronger autoregulatory responses. (A) SSARI (2.5-second segments) distribution profile in intact control rats and (B) the similar effects of ambient BP on mean SSARI (2.5-second segments) in intact and RK-NX rats, and the abrogation of these effects by amlodipine in both groups. *P<0.001 versus intact; †P<0.01 effect of amlodipine versus respective intact and RK-NX rats before amlodipine; δP<0.002 versus respective intact and RK-NX rats during ambient systolic BP of 90–130 mm Hg.
The SSARI Methodology Demonstrates Differences in the AR Response between the Conscious and the Anesthetized State in the Same Rats
Figure 5 presents the SSARI data obtained during conscious recordings and step-AR studies under anesthesia from additional control and RK-NX rats. Anesthesia slows the AR response in rats with both preserved and impaired AR, with complete compensation (SSARI of approximately 0) taking approximately 60 seconds versus approximately 10 seconds in controls rats. More strikingly, the persistent AR impairment seen in RK-NX and RK-NX plus amlodipine rats under anesthesia is not seen in the conscious state.
Figure 5.
Complete autoregulatory compensation of RBF is achieved in conscious but not anesthetized rats. Illustration of the effect of anesthesia on autoregulatory responses, as assessed by the SSARI methodology in the same rats with preserved (control rats with intact renal mass) and impaired AR (RK-NX rats with or without amlodipine) during conscious recordings and after mechanically produced step-BP changes under anesthesia, as previously described. Anesthesia slows the normal autoregulatory responses in intact control rats as indicated by the time to complete autoregulatory compensation (SSARI of approximately 0) of approximately 60 seconds versus approximately 10 seconds in the conscious state. The persistent impairment in AR (reduced magnitude of the AR response) seen under anesthesia in RK-NX rats with or without amlodipine is not observed during the conscious state in these same rats, and complete compensation is achieved although at a slower rate than in control rats with intact renal mass (20 versus 10 seconds). *P<0.006 maximum anesthetized versus conscious in RK-NX and RK-NX plus amlodipine rats for all segment lengths of 1 second or longer. The SEM bars and statistical significance are not shown for SSARI calculated for segment lengths of 2.5 seconds or smaller, for clarity of illustration.
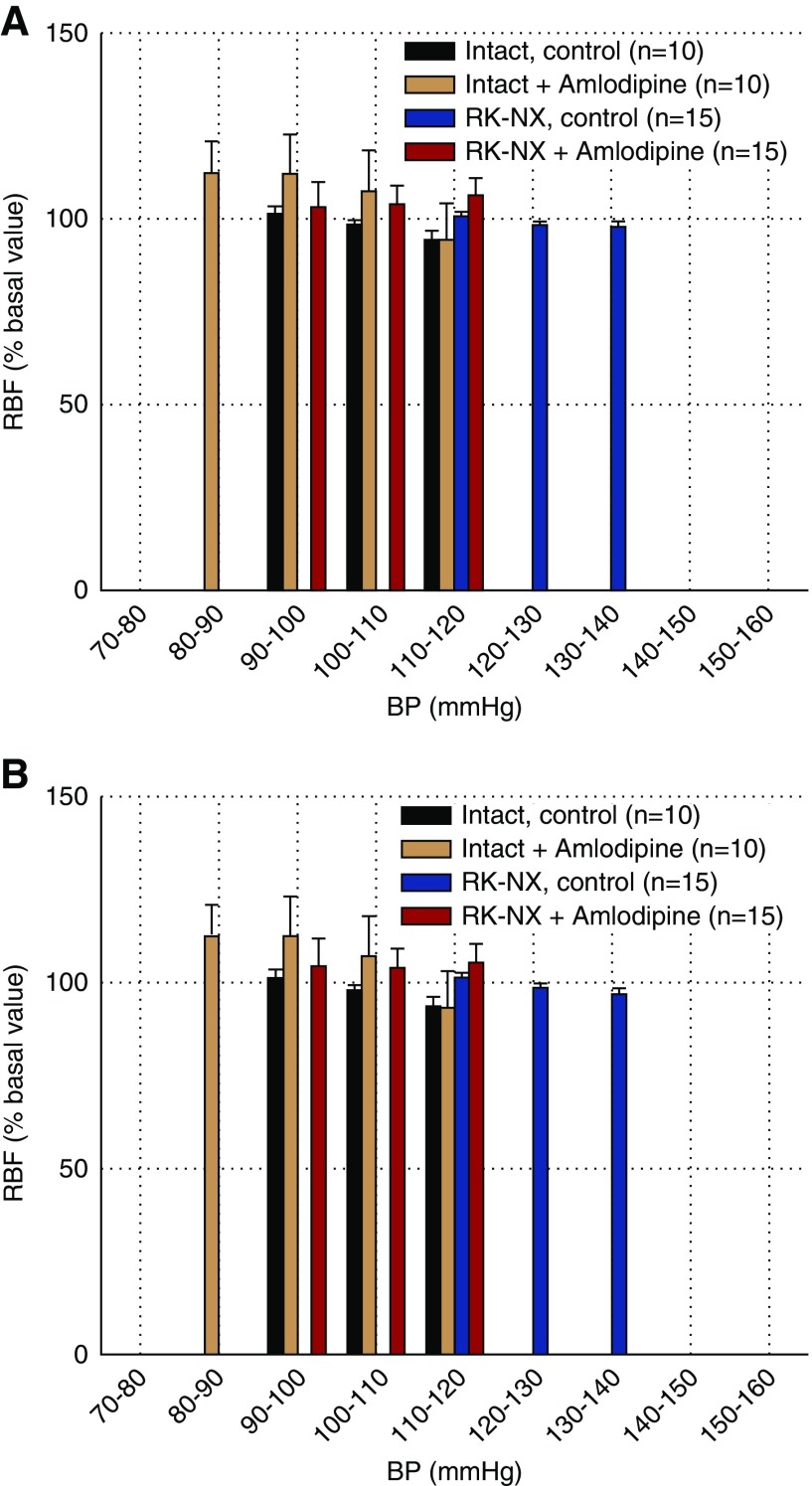
Overall RBF Stability Is Maintained in Conscious Animals during Ambient BP Changes despite Impaired AR by as yet Unidentified Mechanisms
RBF exhibited an overall stability with only limited variation across a large range of ambient BP changes in both control intact and RK-NX rats as illustrated in Figure 6, A and B. Furthermore, the ability to maintain such stability is not altered by amlodipine in either group. It is of note that BP and RBF values that are pooled in each bin reflect values from segments that are separate in time (minutes, hours, and even days). These data indicate that the transient RBF changes that occur acutely after BP changes in states of both preserved and impaired renal AR (SSARI analysis) are not detected by the bin analysis methodology and do not compromise the ability to maintain an overall RBF stability in conscious rats. Of note, this stability is maintained in rats with impaired AR despite transfer function analysis that indicates a severe impairment of the myogenic response, particularly in RK-NX rats receiving amlodipine (Supplemental Figures 4 and 5, Supplemental Table 2).
Figure 6.
Ability to maintain overall stability of RBF is maintained in conscious rats despite autoregulatory impairment. Bin analysis of BP–RBF relationships in conscious intact and RK-NX rats before and after amlodipine administration. Illustrated are (A) 2.5-second segments and (B) 10-second segments. Overall RBF stability was maintained in both groups across the entire examined BP range before and after amlodipine as shown by the similarity of data for the 2.5- and 10-second segment bins.
Discussion
BP is fundamentally labile and exhibits spontaneous and often large fluctuations at multiple frequencies in the conscious state in addition to the oscillations due to the heartbeat.16–20,29–31,34–36 Nevertheless, RBF and GFR are maintained relatively constant, through proportionate adjustments in preglomerular resistance by renal AR.5,6,12–20 As PGC changes are also prevented, renal AR concurrently serves a glomeruloprotective function. However, the temporal requirements for maintaining a stable GFR versus glomeruloprotection may differ, although both involve preventing changes in PGC. For instance, it is possible that transient PGC increases may have the potential for barotrauma but yet not compromise the overall GFR stability. Our studies in conscious rats provide important and novel insights into the real-time dynamics of renal AR responses in the mediation of these regulatory and protective functions.
Although AR capacity has classically been defined solely on the basis of the steady-state magnitude of the AR responses (degree of AR compensation achieved),12–20 the magnitude and duration of RBF (and PGC) changes after an acute BP change depend on both the rate (kinetics) of the AR response and its magnitude. Both aspects can be directly assessed after an acute, sustained step-change in renal perfusion pressure is imposed in anesthetized animals.17–20,32,33,44–46 Such assessments under anesthesia have the merit of describing the physiology of the “pure” AR response to a BP change in the absence of other non-BP inputs, but are limited in characterizing its operational dynamics in the conscious state with continuously fluctuating pressures and other concurrent vascular inputs.16–20,30,31,33–36 By contrast, the SSARI methodology, although based on inference, may nevertheless provide insights into the potential dynamics of BP transmission by analyzing the hemodynamic responses associated with BP change events from an autoregulatory pressure transmission perspective. As expected, the hemodynamic responses to individual BP fluctuations in the conscious rat exhibit a much wider distribution than the more uniform AR responses seen after step-BP changes under anesthesia. Given the myriad concurrent neurohormonal and metabolic signals that can alter/modulate afferent arteriolar responses to BP changes in the conscious state,16,20,30,34–36,39 such variability is not unexpected. Nevertheless, despite this variability, the mean (median) hemodynamic responses associated with BP change events in conscious rats with intact AR exhibited an impressive similarity to the kinetics and the magnitude of the normal step-AR response under anesthesia.
Notably, this resemblance includes an initial, passive, compliance change-related RBF change at 0.5 seconds after the BP change. This feature has been considered an integral component of the vascular myogenic response and is in the same direction as the BP change.47 It precedes the subsequent active AR response, which is in the opposite direction,16–20,32,33,47 making it unlikely that the BP change and the associated hemodynamic responses are AR-independent, concurrent, covariant events occurring for instance in response to changes in sympathetic activity. Likewise, distinct phases of AR compensation similar to that described under anesthesia20,32,33,44–46 are observed in conscious control rats but occur more rapidly. The initial rapid phase of approximately 50% compensation takes approximately 2.5 seconds instead of 4–7 seconds, whereas the slower subsequent phases during which compensation is completed takes approximately 10 seconds in the conscious state but takes 15–60 seconds under anesthesia.17,20,33,44–46 A modest overshoot phase often follows in both situations.17,20,32,33,46 The enhancing effect of ambient BP on the strength of these responses and its abrogation by amlodipine in both intact and RK-NX is consistent with such interpretations.
However, it is in rats with impaired renal AR that the most striking difference in AR responses are observed between the conscious and anesthetized state. Under anesthesia, such rats fail to achieve complete compensation (both in this and previous studies17,20,28,30–33,38) but do so in the conscious state, although it does take almost twice as long to achieve due to slower AR responses (approximately 20 seconds versus the approximately 10 seconds in conscious control rats). This is even true for the RK-NX rats that have additionally received amlodipine and have autoregulatory index values averaging approximately 1.0 under anesthesia, indicating essentially pressure-passive behavior.9,10,16,31 Of note, even this relatively slower compensation is still within the time frame considered normal for the AR response under anesthesia.17,20,32–33,44–46 Thus, in the conscious state, AR impairment seems to be primarily a phenomenon of impaired kinetics rather than the reduced magnitude observed under anesthesia. Nevertheless, given the frequency of BP fluctuations, the effect of the slower AR responses in states of impaired AR on potential glomerular BP transmission and barotrauma may be considerable as indicated by the larger area under the AR curve, a likely index of the increased PGC exposure. The precise magnitude and duration of such increases in PGC, however, are likely to be determined by the prevailing afferent and efferent arteriolar tone (resistances) in addition to the magnitude of the BP change.4–6,9,10,12,15–17,19,20 In this context, it is of note that on the basis of micropuncture measurements, hypertensive glomerular injury is generally believed to be the consequence of sustained elevations in ambient PGC.4–6,20,21 Although our data do not diminish the potential importance of such increases in ambient PGC, they do suggest that pressure transients may play a larger role in the pathogenesis of hypertensive glomerular injury in states of impaired AR than previously thought. Such data also suggest that in states of impaired AR, achievement of normotension may not per se be sufficient to prevent glomerular exposure to increased pressure transients during normal BP fluctuations, which may contribute to the continued, albeit slower progression of CKD despite adequate BP control.9,48
These studies were not directly addressed to specific renal AR mechanisms, but the rapid kinetics of the normal AR response in conscious rats has implications for the contribution of individual AR mechanisms. It is widely held that renal AR is mediated by the combined and interacting contributions of a faster myogenic and the slower macula densa tubuloglomerular feedback (MD-TGF) mechanism.14–20,30–36,43–46,49 Accordingly, Just and coworkers have interpreted the first and second phases of the step-AR response to represent the myogenic and MD-TGF mechanisms with the third phase representing an unidentified third mechanism.32,33 However, the slow operational characteristics of the MD-TGF system are difficult to reconcile with complete AR compensation seemingly occurring even before the estimated TGF signal generation at the MD.43,44,46,49 Indeed, Moss et al.46 have recently demonstrated complete compensation and the persistence of the first two phases during MD-TGF blockade (ureteral occlusion plus mannitol diuresis), suggesting that both initial phases may represent myogenic response components. Of interest, such MD-TGF blockade did abolish the modest AR overshoot (>100% AR compensation), indicating that it may represent a MD-TGF effect. The timing of the overshoot phase in these studies and its abrogation after amlodipine is consistent with such interpretations, given that calcium channel blockade impairs both myogenic and MD-TGF responses.14–20,32,33,38,39 Thus, on the basis of the differences in afferent signals as well as temporal and kinetic characteristics, we and others have suggested that the myogenic and MD-TGF mechanisms may subserve separate and distinct functions.16,18,19,45,46,49,50 The rapid myogenic response may be primarily protective, whereas the slower MD-TGF mechanisms may act to stabilize MD fluid delivery and GFR.
A major finding of these studies was the ability of conscious rats to maintain overall RBF stability and to restore RBF back to baseline after BP changes, albeit more slowly, even when the known renal AR mechanisms are impaired. These data challenge the widely accepted paradigm that the known renal AR mechanisms are the sole mediators of the RBF stability in response to ambient BP changes.14,17,20,30,32–35,45,46,49 That such stability can be maintained even with severe AR impairment, as in RK-NX rats additionally receiving CCBs, suggests its mediation by other mechanosensitive pathways that are independent of voltage-gated calcium channels.20,47 Such an interpretation is supported by the achievement of complete compensation despite the severe impairment of the myogenic response seen by transfer function analysis in RK-NX rats receiving amlodipine. Moreover, the time course of completion of AR compensation occurring between 10 and 20 seconds was similar in all groups with impaired AR despite the likelihood that the mechanisms responsible for AR impairment are different after RK-NX versus amlodipine, as indicated by the differences in transfer functions and the differential sensitivity to ambient BP. Similarly, the differences in the severity of myogenic transfer function impairment or the parallel differences in the step-AR compensation under anesthesia do not seem to differentially affect this slower AR compensation phase in conscious rats. Our data also suggest that these relatively slower AR backup mechanisms may be largely suppressed in the anesthetized state, hindering their recognition. However, plausible rationale support the presence of such backup stabilizers of renal hemodynamics during states of impaired AR.16 For instance, if the normal AR mechanisms were to be solely responsible for preventing chronic BP elevations from altering ambient RBF and GFR as has been postulated, differences in ambient BP would be expected to result in differences in ambient RBF and GFR in states of impaired AR. But this is not observed.16,30,31,40 Even more compellingly, antihypertensive therapy in states of impaired AR would be predicted to reduce ambient RBF and GFR, similar to what is observed with BP lowering during step-AR studies. Yet, such is not observed typically in either CKD models or in human CKD, consistent with the postulated role of backup hemodynamic stabilizers.16
In summary, our studies in conscious rats indicate that protection against glomerular transmission of BP fluctuations normally occurs over rapid time scales (5–10 seconds). In states of impaired AR, the responses are significantly slower, but unlike under anesthesia, complete compensation is achieved, although it takes almost twice as long (approximately 20 seconds). Given that this phenomenon is observed even with severe AR impairment after calcium channel blockade, these data suggest the presence of additional, slower anesthesia-sensitive but as yet unidentified mechanosensitive mechanism(s) independent of voltage-gated calcium channels potentially operative in the conscious state. Our studies indicate that these mechanisms may be critical to maintaining RBF and GFR stability during episodic or sustained BP changes in states of AR impairment.
Disclosures
Dr. Bidani reports grants from National Institutes of Diabetes and Digestive and Kidney Diseases, during the conduct of the study; and grants from Bayer AG, outside the submitted work. Dr. Griffin reports grants from National Institutes of Health and the Department of Veterans Affairs. Dr. Polichnowski reports grants from American Heart Association, American Society of Nephrology Foundation for Kidney Research, National Kidney Foundation of Illinois, and Veterans Administration, during the conduct of the study. All remaining authors have nothing to disclose.
Funding
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases grants DK-40426 (to Dr. Bidani) and DK-61653 (to Dr. Griffin), by a Merit Review Award (to Dr. Griffin) and Career Development Awards from the Office of Research and Development of the Department of Veterans Affairs (1IK2BX001285 to Dr. Polichnowski), and from the National Kidney Foundation of Illinois (to Dr. Polichnowski). Dr. Polichnowski is currently supported by a Carl Gottschalk Research Scholar Grant from the American Society of Nephrology Foundation for Kidney Research and the American Heart Association (17AIREA33660433).
Supplementary Material
Acknowledgments
Dr. Bidani, Dr. Griffin, Dr. Williamson, and Dr. Polichnowski designed the study; Dr. Licea-Vargas and Dr. Polichnowski carried out the experiments; Dr. Bidani, Dr. Griffin, Dr. Polichnowski, Dr. Williamson, and Mr. Long analyzed the data; Dr. Kliethermes performed the statistical analysis; Mr. Long, Dr. Williamson, Dr. Polichnowski, and Dr. Griffin made the figures; Dr. Bidani, Dr. Griffin, Dr. Polichnowski, and Dr. Williamson drafted and revised the manuscript; all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019070718/-/DCSupplemental.
Supplemental Figure 1. BP and RBF recordings from both conscious (A and B) and anesthetized (C) RK-NX rats to illustrate the methodology for SSARI analysis.
Supplemental Figure 2. The effect on calculated SSARI of BP increases versus decreases or of a change in MAP of >10 minutes versus >5 mm Hg between adjacent segments.
Supplemental Figure 3. A comparison of SSARI calculated in the standard fashion versus when SSARI analysis was restricted to 10-second segments that exhibited minimal BP variation within the segment after a change in BP of at least 5 mm Hg from a preceding segment of at least 5 seconds duration (step-like SSARI).
Supplemental Figure 4. Transfer function analysis of the relationship between BP (input) and RBF (output) in intact control and RK-NX rats before and after amlodipine.
Supplemental Figure 5. Illustration of the flat transfer functions in an RK-NX rat after amlodipine.
Supplemental Table 1. Number of segments used for SSARI calculations for BP change of at least 10 mm Hg between adjacent segments and the mean BP change for each segment length.
Supplemental Table 2. Transfer function parameters of relevance to the operational characteristics of the myogenic mechanism of renal AR.
References
- 1.Brenner BM, Meyer TW, Hostetter TH: Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307: 652–659, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Klahr S, Schreiner G, Ichikawa I: The progression of renal disease. N Engl J Med 318: 1657–1666, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al.: Blood pressure and end-stage renal disease in men. N Engl J Med 334: 13–18, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Brenner BM, Lawler EV, Mackenzie HS: The hyperfiltration theory: A paradigm shift in nephrology. Kidney Int 49: 1774–1777, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Bidani AK, Griffin KA: Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension 44: 595–601, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Griffin KA: Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension 70: 687–694, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, et al. ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 8.KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2: 337–414, 2013 [Google Scholar]
- 9.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA: Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 22: 1–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin KA, Bidani AK: Progression of renal disease: Renoprotective specificity of renin-angiotensin system blockade. Clin J Am Soc Nephrol 1: 1054–1065, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Mori T, Cowley AW Jr.: Role of pressure in angiotensin II-induced renal injury: Chronic servo-control of renal perfusion pressure in rats. Hypertension 43: 752–759, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Robertson CR, Deen WM, Troy JL, Brenner BM: Dynamics of glomerular ultrafiltration in the rat. 3. Hemodynamics and autoregulation. Am J Physiol 223: 1191–1200, 1972 [DOI] [PubMed] [Google Scholar]
- 13.Arendshorst WJ, Finn WF, Gottschalk CW: Autoregulation of blood flow in the rat kidney. Am J Physiol 228: 127–133, 1975 [DOI] [PubMed] [Google Scholar]
- 14.Navar LG: Renal autoregulation: Perspectives from whole kidney and single nephron studies. Am J Physiol 234: F357–F370, 1978 [DOI] [PubMed] [Google Scholar]
- 15.Schnermann J, Briggs JP: Interaction between loop of Henle flow and arterial pressure as determinants of glomerular pressure. Am J Physiol 256: F421–F429, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Loutzenhiser R, Griffin K, Williamson G, Bidani A: Renal autoregulation: New perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cupples WA, Braam B: Assessment of renal autoregulation. Am J Physiol Renal Physiol 292: F1105–F1123, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Loutzenhiser R, Bidani A, Chilton L: Renal myogenic response: Kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R: Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlström M, Wilcox CS, Arendshorst WJ: Renal autoregulation in health and disease. Physiol Rev 95: 405–511, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson JL: Renal disease caused by hypertension. In: Heptinstall’s Pathology of the Kidney, edited by Jennette JC, Olson JL, Schwartz MM, Silva FG. 6th ed II. Philadelphia, PA, Lippincott Williams & Wilkins, pp 937–990, 2006 [Google Scholar]
- 22.Hill GS: Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens 17: 266–270, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Byrom FB: The Hypertensive Vascular Crisis: An Experimental Study. Heineman Monograph, London, United Kingdom, Pitman Press, 1969 [Google Scholar]
- 24.Arendshorst WJ: Autoregulation of renal blood flow in spontaneously hypertensive rats. Circ Res 44: 344–349, 1979 [DOI] [PubMed] [Google Scholar]
- 25.Griffin KA, Churchill PC, Picken M, Webb RC, Kurtz TW, Bidani AK: Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am J Hypertens 14: 311–320, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA: Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol Renal Physiol 289: F305–F313, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Griffin KA, Polichnowski A, Litbarg N, Picken M, Venkatachalam MA, Bidani AK: Critical blood pressure threshold dependence of hypertensive injury and repair in a malignant nephrosclerosis model. Hypertension 64: 801–807, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bidani AK, Schwartz MM, Lewis EJ: Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol 252: F1003–F1010, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Bidani AK, Griffin KA, Picken M, Lansky DM: Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol 265: F391–F398, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA: “Step” vs. “dynamic” autoregulation: Implications for susceptibility to hypertensive injury. Am J Physiol Renal Physiol 285: F113–F120, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Griffin KA, Picken MM, Bidani AK: Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest 96: 793–800, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Just A, Arendshorst WJ: Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol 285: R619–R631, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Just A: Mechanisms of renal blood flow autoregulation: Dynamics and contributions. Am J Physiol Regul Integr Comp Physiol 292: R1–R17, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Holstein-Rathlou NH, Marsh DJ: Renal blood flow regulation and arterial pressure fluctuations: A case study in nonlinear dynamics. Physiol Rev 74: 637–681, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH: Dynamic autoregulation and renal injury in Dahl rats. Hypertension 30: 975–983, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Pires SL, Barrès C, Sassard J, Julien C: Renal blood flow dynamics and arterial pressure lability in the conscious rat. Hypertension 38: 147–152, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Loutzenhiser R, Epstein M: Effects of calcium antagonists on renal hemodynamics. Am J Physiol 249: F619–F629, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Navar LG, Champion WJ, Thomas CE: Effects of calcium channel blockade on renal vascular resistance responses to changes in perfusion pressure and angiotensin-converting enzyme inhibition in dogs. Circ Res 58: 874–881, 1986 [DOI] [PubMed] [Google Scholar]
- 39.Griffin KA, Hacioglu R, Abu-Amarah I, Loutzenhiser R, Williamson GA, Bidani AK: Effects of calcium channel blockers on “dynamic” and “steady-state step” renal autoregulation. Am J Physiol Renal Physiol 286: F1136–F1143, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Griffin KA, Picken M, Bidani AK: Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol 4: 2023–2031, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Polichnowski AJ, Griffin KA, Long J, Williamson GA, Bidani AK: Blood pressure-renal blood flow relationships in conscious angiotensin II- and phenylephrine-infused rats. Am J Physiol Renal Physiol 305: F1074–F1084, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polichnowski AJ, Griffin KA, Picken MM, Licea-Vargas H, Long J, Williamson GA, et al.: Hemodynamic basis for the limited renal injury in rats with angiotensin II-induced hypertension. Am J Physiol Renal Physiol 308: F252–F260, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffin K, Polichnowski A, Licea-Vargas H, Picken M, Long J, Williamson G, et al.: Large BP-dependent and -independent differences in susceptibility to nephropathy after nitric oxide inhibition in Sprague-Dawley rats from two major suppliers. Am J Physiol Renal Physiol 302: F173–F182, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young DK, Marsh DJ: Pulse wave propagation in rat renal tubules: Implications for GFR autoregulation. Am J Physiol 240: F446–F458, 1981 [DOI] [PubMed] [Google Scholar]
- 45.Gannon KP, McKey SE, Stec DE, Drummond HA: Altered myogenic vasoconstriction and regulation of whole kidney blood flow in the ASIC2 knockout mouse. Am J Physiol Renal Physiol 308: F339–F348, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moss NG, Gentle TK, Arendshorst WJ: Modulation of the myogenic mechanism: Concordant effects of NO synthesis inhibition and O2- dismutation on renal autoregulation in the time and frequency domains. Am J Physiol Renal Physiol 310: F832–F845, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Hill MA: Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Bidani AK, Griffin KA, Epstein M: Hypertension and chronic kidney disease progression: Why the suboptimal outcomes? Am J Med 125: 1057–1062, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniels FH, Arendshorst WJ, Roberds RG: Tubuloglomerular feedback and autoregulation in spontaneously hypertensive rats. Am J Physiol 258: F1479–F1489, 1990 [DOI] [PubMed] [Google Scholar]
- 50.John E: Hall; glomerular filtration, renal blood flow and their control. In: Guyton and Hall Textbook of Medical Physiology, 13th John Hall, Ph.D. Ed., Philadelphia, PA, Elsevier Inc, 2016, pp 344–345 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.