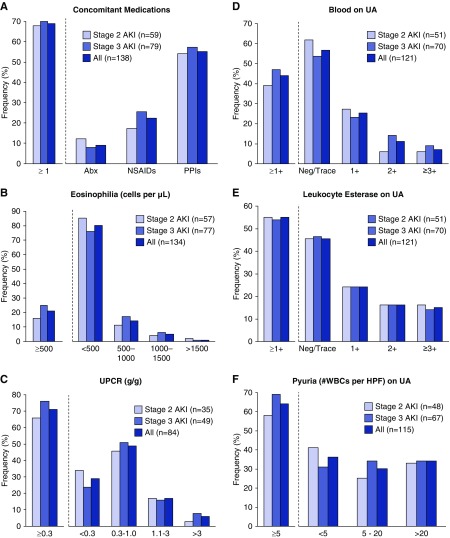
Figure 2.
Clinical features of ICPi-AKI, stratified by AKI severity. (A) The frequency of concomitant potential TIN-causing medications taken within 2 weeks preceding ICPi-AKI. (B–F) The distribution of eosinophilia, proteinuria, dipstick hematuria, leukocyte esterase, and pyuria in patients with ICPi-AKI, respectively. Abx, antibiotic; HPF, high-power field; Neg, negative; UA, urinalysis; UPCR, urine protein-to-creatinine ratio; WBCs, white blood cells.