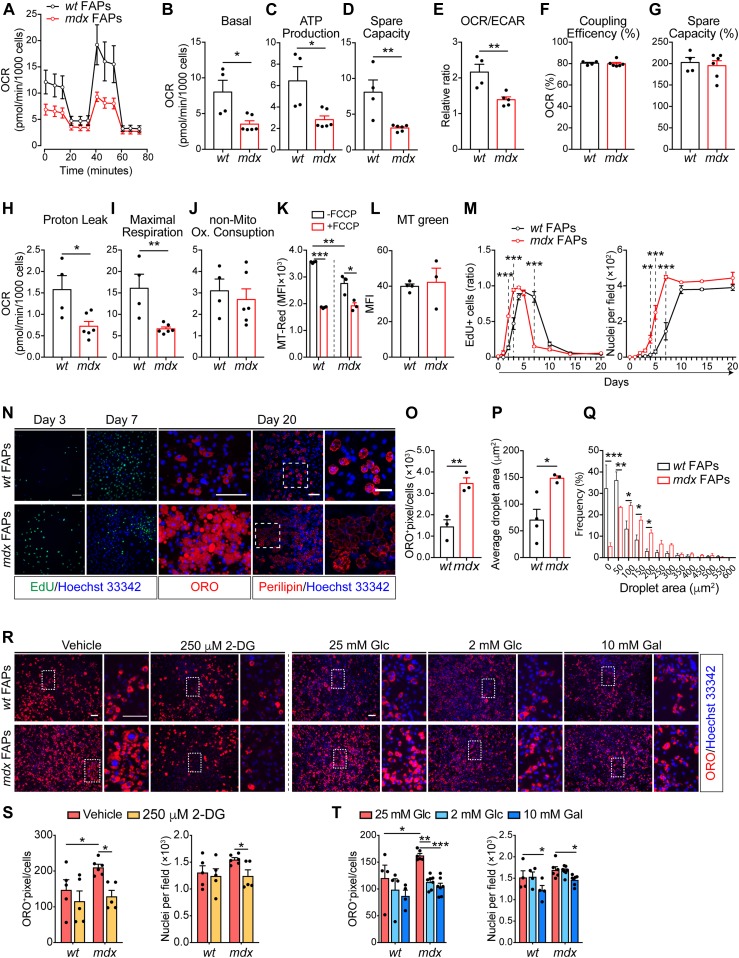
Figure 2. Dystrophic fibro/adipogenic progenitor (FAP) mitochondrial dysfunction correlates with an impaired ex vivo proliferation and adipogenic differentiation that can be modulated by metabolic interventions.
(A) Mitochondrial stress test profile of wt and mdx FAPs (from 45-d old wt and mdx mice) by Seahorse analysis. The oxygen consumption rate (OCR) (pmol/min/103 cells) was monitored for 80 min under basal conditions and upon sequential treatment with the mitochondrial inhibitors oligomycin, FCCP, and rotenone/antimycin (wt FAPs n = 4; mdx FAPs n = 6). (B, C, D, E, F, G, H, I, J) Bar graphs representing basal OCR (B), ATP production (C), spare capacity (D), OCR/extracellular acidification rate ratio (E), coupling efficiency (% to the basal OCR) (F), spare capacity (% to the basal OCR) (G), proton leak (H), maximal respiration (I), and non-mitochondrial oxygen consumption (J) obtained by Seahorse Wave Desktop software. Statistical significance was estimated by the t test. (K) Bar graph representing the median fluorescence intensity (MFI) of MitoTracker RED (MT-Red) dye in flow cytometry in basal condition and under uncoupling with 10 μM FCCP, in wt and mdx FAPs (n = 3). Statistical significance was estimated by the t test. (L) Bar graph representing median fluorescence intensity (MFI) of MitoTracker GREEN (MT Green) dye in flow cytometry in basal condition on wt and mdx FAPs (n = 3). Statistical significance was estimated by the t test. (M) EdU labelling and growth curve profile of FAPs purified from 45-d old wt and mdx mice. FAPs were cultured for 20 d (wt n = 3; mdx n = 3). Statistical significance was estimated by two-way ANOVA. (N) Representative EdU (green, 20× magnification; scale bar, 100 μm), Oil Red O (ORO) staining (red, 40× magnification; scale bar, 100 μm), and confocal micrographs of perilipin immunostaining (red, 20× magnification; scale bar, 70 μm) of FAP cells at 3, 7, and 20 d. Nuclei (blue) were revealed with Hoechst 33342. (N, O) Bar graph resenting the adipogenic differentiation index of wt and mdx FAPs calculated as ORO-positive pixels/cell from the panel (N) (n = 3). (N, P) Bar graph representing the average lipid droplet area (μm2) of confocal images in panel (N) (wt n = 4; mdx n = 3). (N, Q) Bar graph representing the frequency distribution of lipid droplet areas of confocal images in panel (N) (wt n = 4; mdx n = 3). Statistical significance was estimated by t test. (R) Representative ORO staining (10× magnification; scale bar, 100 μm) of FAPs from 45-d-old wt and mdx mice. Adipogenic differentiation was obtained by incubating FAPS in adipocyte differentiation medium (ADM) followed by the adipocyte maintenance medium (AMM) in the presence of 25 mM glucose (Glc) supplemented with DMSO (vehicle) or 250 μM 2-deoxyglucose (2-DG). Alternatively, FAPs were differentiated by incubating cells with the opportune differentiation media containing either 25 mM Glc, 2 mM Glc, or 10 mM galactose (Gal). Insets are enlarged views of the dashed areas (scale bar: 100 μm). Nuclei (blue) were revealed with Hoechst 33342. (S) Bar plots reporting the adipogenic index (left) and the average number of nuclei per field (right) for FAPs differentiated in the presence of the vehicle or 250 μM 2-DG treatment. (T) Bar plots reporting the adipogenic index (left) and the average number of nuclei per field (right) for FAPs differentiated in the presence of 25 mM Glc, 2 mM Glc, or 10 mM Gal treatment (wt n = 3; mdx n = 3). Statistical significance was estimated by two-way ANOVA. All data are represented as mean ± SEM and statistical significance is defined as *P < 0.05; **P < 0.01; ***P < 0.001.