Summary
Background
Onychomycosis affects almost 6% of the world population. Topical azoles and systemic antifungal agents are of low efficacy and can have undesirable side effects. An effective, non‐invasive therapy for onychomycosis is an unmet clinical need.
Objective
Determine the efficacy threshold of non‐thermal atmospheric plasma (NTAP) to treat onychomycosis in an in vitro model.
Methods
A novel toe/nail‐plate model using cadaver nails and agarose media inoculated with Candida albicans was exposed to a range of NTAP doses.
Results
Direct exposure of C albicans and Trichophyton mentagrophytes to 12 minutes of NTAP results in complete killing at doses of 39 and 15 kPulses, respectively. Onset of reduced viability of C albicans to NTAP treatment through the nail plate occurs at 64 kPulses with 10× and 100× reduction at 212 and 550 kPulses, respectively.
Conclusions
NTAP is an effective, non‐invasive therapeutic approach to onychomycosis that should be evaluated in a clinical setting.
Keywords: Candida albicans, cold atmospheric plasma, dielectric barrier discharge, fungus, non‐thermal atmospheric plasma, onychomycosis, pulsed electric field, Trichophyton mentagrophytes
1. INTRODUCTION
Onychomycosis, a fungal infection of the nail, afflicts almost 6% of the population worldwide.1 The current treatment strategy includes a combination of oral and topical agents. Terbinafine is the preferred oral therapy, with 38% complete cure rate, but it can be hepatotoxic. Efinaconazole and tavaborole are preferred topical antifungals which require daily application for months, and yield cure rates of up to 17.8% and 9.1%, respectively.2 Recently, pulsed lasers have been used to treat onychomycosis, primarily through a photothermal effect, but efficacy is limited and the treatment is often painful.3
Non‐thermal atmospheric plasma (NTAP) treatment technologies have been investigated for almost two decades resulting in several medical applications.4, 5 They have demonstrated broad‐spectrum anti‐microbial effects and can be used to disinfect biotic (eg wounds) and abiotic surfaces,6, 7 and treat cancerous tumours.8 One study demonstrated that NTAP inactivates causative agents for onychomycosis (Trichophyton rubrum and Microsporum canis); however, these experiments were done in the absence of human nails.9 A clinical trial (pilot study) to treat onychomycosis using NTAP with a manually scanned electrode was recently conducted with significant success.10
The plasma environment responsible for treatment efficacy includes pulsed electric field (which couples strongly through the nail plate), induced current through the tissue, and some moderate local heating. The plasma also generates active chemical species that interact with treated surfaces and generate solvated long‐lived reactive species in the liquid.11 There is evidence that reactive nitrogen species penetrate through tissues12, 13, 14 which is important for the treatment of onychomycosis where the dose must be delivered through the nail plate.
Non‐thermal atmospheric plasma is created at normal atmospheric pressure (~100 kPa), where ions and neutral species have not reached thermal equilibrium with electrons, and the gas temperature is <60°C. One method of generating NTAP is the dielectric barrier discharge (DBD)15 where a dielectric layer is placed in the gap between electrodes to which a time‐varying (RF or pulsed) high voltage is applied. The time‐averaged current through a DBD is inherently limited due to the presence of the dielectric.
In this paper, we first establish that both Trichophyton mentagrophytes, a prominent agent of toenail infections, and Candida albicans, a causative agent of fingernail onychomycosis, are killed when NTAP is created directly above cultures placed on agar. We then describe the toe/nail‐plate in vitro model and the efficacy of NTAP on the viability of C albicans shielded by this nail plate. Treatment conditions that lead to over 2‐log reduction in fungal viability are identified.
2. MATERIALS AND METHODS
2.1. Fungal strains
Candida albicans (ATCC 24433) and T mentagrophytes (ATCC MYA‐4439) were plated on 1.5% YPD agar (Difco) and 1.5% Sabouraud‐Dextrose (S‐D) agar (Difco), respectively. After incubation at 30°C (2‐3 days for C albicans, 5‐7 days for T mentagrophytes), several colonies of each species were stained by lactophenol cotton blue dye and microscopically examined for hyphal morphology. C albicans isolates were mostly hyphal, which is associated with human infections, and all T mentagrophytes isolates showed typical dermatophyte morphology with hyphae and microconidia and macroconidia. A single colony of each fungal species was selected and grown in liquid YPD and S‐D media, respectively, and 0.2 mL aliquots were frozen in 30% glycerol at −80°C. Each frozen aliquot was used only once for each experiment.
2.2. Nails and nail model
An in vitro toe/nail‐plate model was developed (MOE Medical Devices) using 2% agar nail moulds as “toes” covered with sterilized, overt‐onychomycosis‐free hallux toenails from human cadavers (Science Care). Cadaver nail plates have substantially the same physical and electrical properties as those of living patients. YPD and S‐D broth with 2% agar have conductivity and permittivity of 1 S/m and 80, respectively, which approximates the toe without the nail plate (0.3‐1.0 S/m and 45‐200).16, 17 A sterile mould of the underside of each nail plate was created as follows: (a) pour agar into a Petri dish; (b) place a clean, sterile nail plate onto the agar while it is cooling and confirm there are no air bubbles between the nail plate and the agar; (c) remove excess agar from the top and sides of the nail plate after the agar hardens. After each experiment, the nails were rinsed with water, cleaned with 70% alcohol, autoclaved in distilled water and re‐used.
2.3. Preparation of C albicans cell suspensions
Liquid cultures (5 mL) of C albicans were inoculated with 100 μL of frozen culture, grown for 3 days at 34°C and diluted to OD595 = 0.1 (~5 × 106 colony‐forming units per mL (CFU/mL)). A 10 μL (~5 × 104 CFU) suspension was used to inoculate nail mould samples.
2.4. Preparation of T mentagrophytes suspensions
Liquid cultures (5‐7 days) of T mentagrophytes in S‐D broth were vortexed and passed through a 23G tuberculin syringe to fragment hyphae. Serial dilutions in S‐D broth were made from a suspension containing ~103 CFU/mL, and 10 μL of this suspension (~10 CFU) was used for each sample inoculant to be exposed to NTAP.
2.5. Generation and application of non‐thermal atmospheric plasma
Non‐thermal atmospheric plasma was delivered to toenails with single‐use patch electrodes consisting of flexible conductive copper mesh surrounded by medical‐grade rubber insulation (Figure 1C). The active area of the electrode has a square array of rubber bumps which provide the necessary separation between the nail plate and dielectric surface of the electrode so as to create a DBD in the air between these two surfaces. The patches are connected to a generator producing high‐voltage pulses with amplitude sufficient to initiate a discharge.
Figure 1.
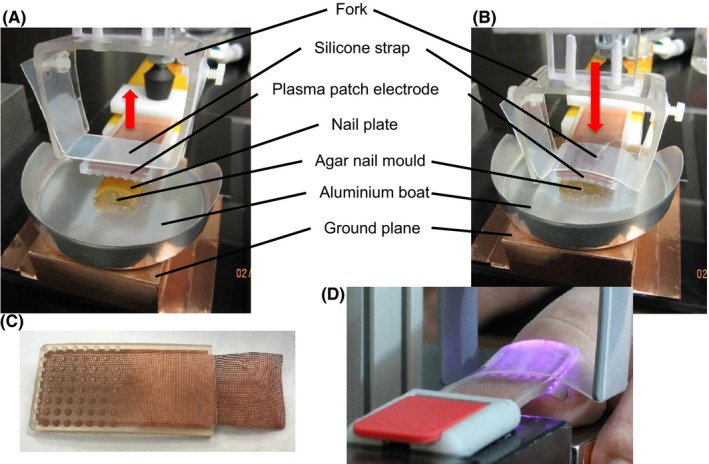
Plasma treatment experimental setup (A) before application of the plasma patch electrode and (B) with conformal application of the electrode to the cadaver nail plate on the agar nail mould. Plasma patch electrode (C) removed from the system and (D) installed in a treatment apparatus showing plasma discharge on patient hallux under the electrode
The generator output into an impedance‐matched load is a monopolar pulse of fixed rise time (<20 ns) and pulse width (<100 ns). The nominal peak output voltage amplitude (AMP) is adjustable from 5 to 20 kV. The pulse repetition frequency (PRF) can be varied from 1 Hz to 1 kHz. Other treatment parameters include uninterrupted treatment time (T ON), number of uninterrupted treatments (N), and rest time between uninterrupted treatments (T OFF). We use the short‐hand notation SEQ = N × T ON(T OFF) to indicate the treatment sequence. The total dose (D) is the number of delivered pulses given by D = N × T ON × PRF.
2.6. Protocol for C albicans sensitivity testing using the nail‐agar mould model
For each experiment, place the agar nail mould into an aluminium boat, remove the nail plate from the mould, and inoculate the agar mould with 10 μL of culture of the desired microorganism;
Reposition nail plate on the mould, place aluminium boat onto the ground plane with mould and nail plate aligned directly under the plasma patch electrode, and secure electrode conformally to the nail plate with the silicone strap (Figures 1A and 1B);
Pulse with indicated AMP, PRF, and SEQ;
Release patch electrode from nail plate and remove nail plate from mould;
(a) Wash nail plate in 5 mL sterile water, centrifuge supernatant 10 minutes at 10 600 g, suspend pellet in 1 mL sterile water, and assay for viable microorganisms by plating 100 μL aliquots on three YPD agar plates and culture for 3 days at 30°C; (b) place agar mould (post‐treatment) into a Petri dish with YPD agar and culture for 3 days at 30°C;
(a) Count colonies on agar plates and calculate the number of CFUs in the nail wash; (b) assess survival on agar moulds.
(The combination of two assessment methods allows detection of viable microorganisms either remaining on the agar mould or adhering to the nail.)
2.7. Protocol for exposure of C albicans and T mentagrophytes to NTAP on agar without nails
Comparison of sensitivities to NTAP without the protective cover of a nail was performed on solid YPD agar and S‐D agar slabs. A 10 μL drop of fungi suspension, prepared as previously described, was inoculated on the surface of an agar slab the size of the patch electrode active area and exposed to NTAP.
2.8. Temperature measurements in the nail‐agar mould model
Temperature measurements were made on the surface of the agar directly under the nail plate during NTAP treatment with a floating, shielded, miniature probe. The probe was a type‐K thermocouple with continuous stainless steel (304SS) sheath connected to a logger with 0.5°C resolution and ±0.5°C accuracy. The continuous sheath was necessary to prevent disruption of the logger due to plasma noise. The electrically floating probe tip of 0.010″ (254 μm) diameter presented an insignificant thermal load and minimal electrical perturbation to the nail‐agar system exposed to NTAP.
2.9. Statistical analysis of dose‐response for C albicans sensitivity testing
A dose‐response curve was generated, where dose (D) is in kPulses and response is kill ratio (R). Results of each experiment (Ri) were plotted along with the average () for multiple trials (n) at each dose and 95% confidence intervals (CI).18 The CI were calculated using CI = (t .95/√n)∙SD, where t.95 are the critical values (percentiles) for the 95% one‐sided t‐distribution and SD is standard deviation.
The Ri, , and CI were transformed to log reduction (LR) values using
| (1) |
and re‐plotted in this form to show response at high doses. CI was calculated using Equation 1 to transform + CI and − CI for each dose.
All samples (Ri) were fit to the variable‐slope sigmoidal dose‐response curve given by
| (2) |
where D 50 is the dose yielding 50% response or LogEC50, and h is the Hill slope.19 The solid curves shown in Figures 5A and 5B are the least‐squares best fit to Equation 2 and the transform of this best‐fit equation using Equation 1, respectively. The least‐squares fit of Ri to Equation 2 properly weighs the transition at R = 50% but underestimates R at high dose rates. Consequently, the data were fit to the log‐reduction–transformed dose‐response curve (substitute Equation 2 in Equation 1). The result is shown as dashed curves in Figure 5.
Figure 5.
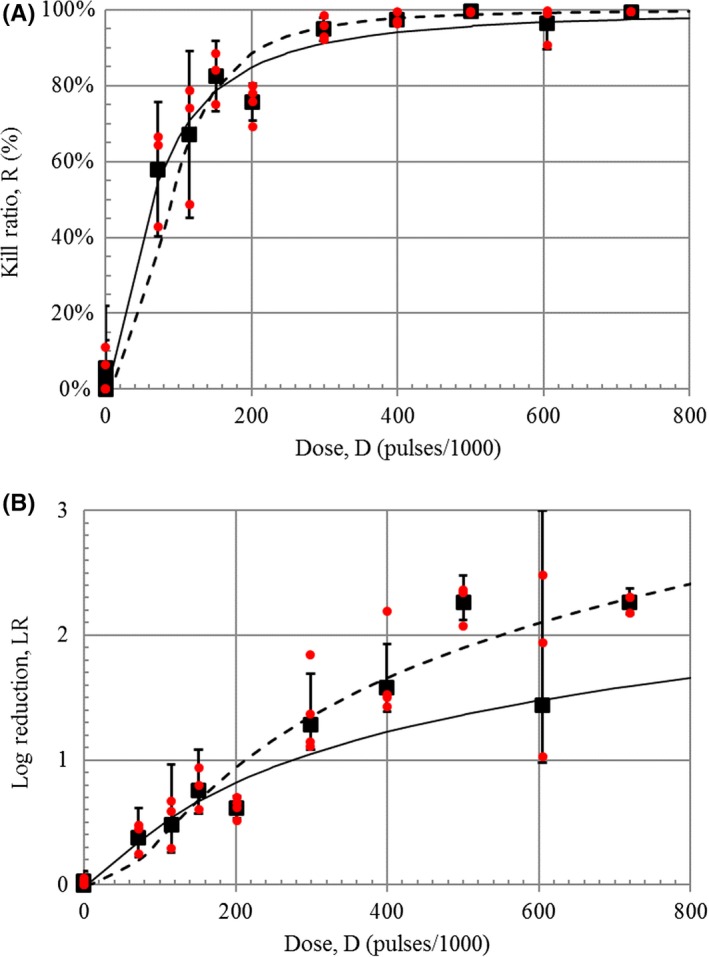
Dose‐response curve for plasma treatment of nail plate inoculated with Candida albicans plotted as (A) kill ratio and (B) log reduction vs dose for fixed intensity (AMP = 18.5 kV) and treatment sequence (SEQ = 3×4′(1′)). Individual independent measurements, Ri, are plotted as small closed red circles ( ), and average values, , are shown as closed black squares (■) with 95% confidence interval error bars (CI = (t
.95/√n)∙SD, where n = 3 or 4). The solid curve is the least‐squares best‐fit (r
2 = .922) variable‐slope sigmoidal dose‐response with D
50 = 63.6 kPulses and h = 1.50. Dashed curve is best fit to sigmoidal dose‐response transformed to log reduction
), and average values, , are shown as closed black squares (■) with 95% confidence interval error bars (CI = (t
.95/√n)∙SD, where n = 3 or 4). The solid curve is the least‐squares best‐fit (r
2 = .922) variable‐slope sigmoidal dose‐response with D
50 = 63.6 kPulses and h = 1.50. Dashed curve is best fit to sigmoidal dose‐response transformed to log reduction
3. RESULTS
3.1. Antifungal effects of direct exposure of C albicans and T mentagrophytes to NTAP
Direct treatment with NTAP of C albicans culture on an agar mould without a nail plate resulted in strong antifungal effects within 1 minute of exposure at AMP = 18.5 kV and PRF = 840 Hz, as compared to untreated control (Figure 2). Continuous treatment durations of more than 4 minutes at the same NTAP parameters resulted in no detectible fungal colony growth. (Data not shown.)
Figure 2.

Images of Candida albicans and Trichophyton mentagrophytes cultures treated with plasma directly on agar without nails. Treatment conditions for both organisms were AMP = 18.5 kV, SEQ = 1 × 1′(0′) and PRF as indicated. PRF = 0 Hz is untreated control
We assessed if the result was affected by microorganisms sticking to the electrode. Placing the electrode onto the inoculated agar slab without pulsing results in about 0.3% of the C albicans CFUs adhering to the electrode (from viable CFUs in electrode washes). Following 1 minute of plasma treatment, electrode wash showed no growth.
Examination of the effects of direct exposure of C albicans to increasing doses of NTAP revealed that ≥39 kPulses were required for killing all microorganisms in the 104 CFU inoculum (Figure 2). Direct exposure of T mentagrophytes to increasing doses of NTAP demonstrated complete killing for doses ≥15 kPulses (Figure 2).
3.2. Measurement of nail‐agar mould (nail bed) temperature
The temperature between the nail‐plate and agar mould was measured to determine if treatment with NTAP produces temperatures sufficient to kill fungus by thermal effects alone. A typical measurement vs time is shown in Figure 3A (inset). The system reaches thermal equilibrium in about four minutes, typical for all pulse generator settings and agar/nail‐plate samples. At this level of treatment, the system does not fully recover to room temperature during the two‐minute pause; however, the same maximum temperature of 45°C is reached during each of the three successive uninterrupted treatments.
Figure 3.
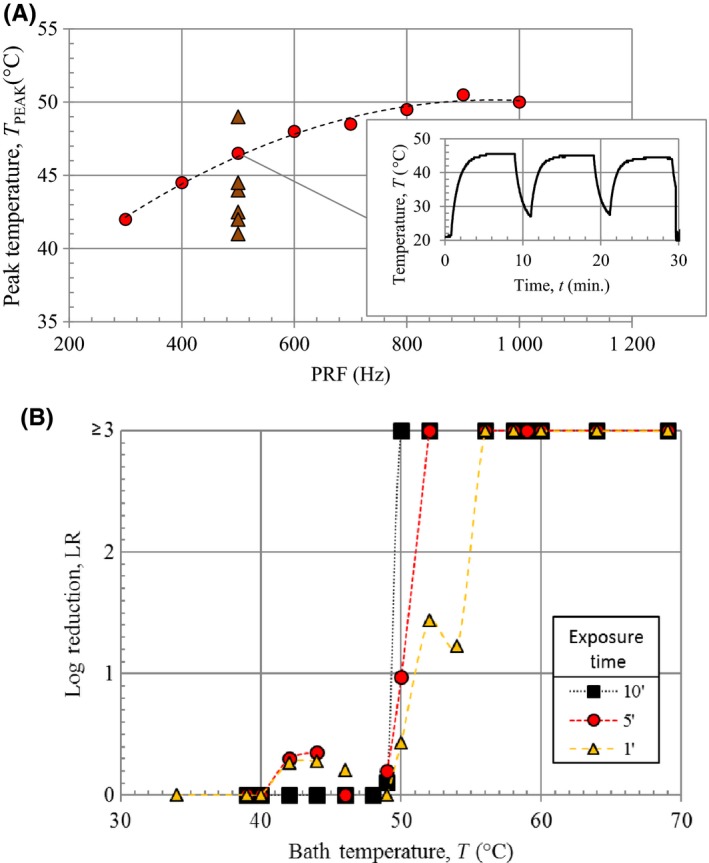
Temperature measurements. (A) Peak temperature measured under the nail plate in vitro during plasma treatment vs dose rate, and (A‐inset) measured temperature vs time at PRF = 500 Hz. All data collected at AMP = 18 kV and SEQ = 3 × 8′(2′). Temperature with increasing dose plotted as red circles ( ) and six additional nail plate samples tested at PRF = 500 Hz plotted as brown triangles (
) and six additional nail plate samples tested at PRF = 500 Hz plotted as brown triangles ( ). (B) Average log reduction of viability vs temperature for Candida albicans exposed to an elevated temperature bath for 1, 5, and 10 min
). (B) Average log reduction of viability vs temperature for Candida albicans exposed to an elevated temperature bath for 1, 5, and 10 min
The temperature measurement was repeated for different PRF settings. Maximum temperature vs PRF is represented in Figure 3A. Peak temperatures increase from 43°C to a maximum of about 51°C as the PRF increases from 300 to 1000 Hz. Several measurements were made with different agar/nail‐plate samples at fixed PRF (500 Hz) to measure the variation in heating. The resulting range in temperature is 41‐48°C.
3.3. Measurement of thermotolerance of C albicans
Candida albicans samples were exposed to a range of temperatures (34‐69°C) in a constant temperature water bath. For exposure times of 1, 5, and 10 minutes, complete killing was observed for temperatures ≥56, ≥52, and ≥50°C, respectively (Figure 3B). Significant reduction in viability was observed from 50‐54°C with virtually no reduction in viability below 50°C for exposure times up to 10 minutes. This is within 1°C of the maximum temperature observed during agar/nail‐plate NTAP treatment. Therefore, NTAP‐induced heating alone is insufficient to eliminate C albicans.
3.4. Exposure of C albicans under cadaver nails to NTAP
The nail‐agar mould model inoculated with C albicans was exposed to NTAP for increasing doses with pulse generator settings of AMP = 18.5 kV, SEQ = 3 × 4′(1′), and PRF from 0 to 1 kHz (Figure 4). Two to four independent experiments were performed for each dose. Near‐complete fungal elimination is observed for doses ≥605 kPulses which is about 15 times higher than that of direct exposure to NTAP.
Figure 4.
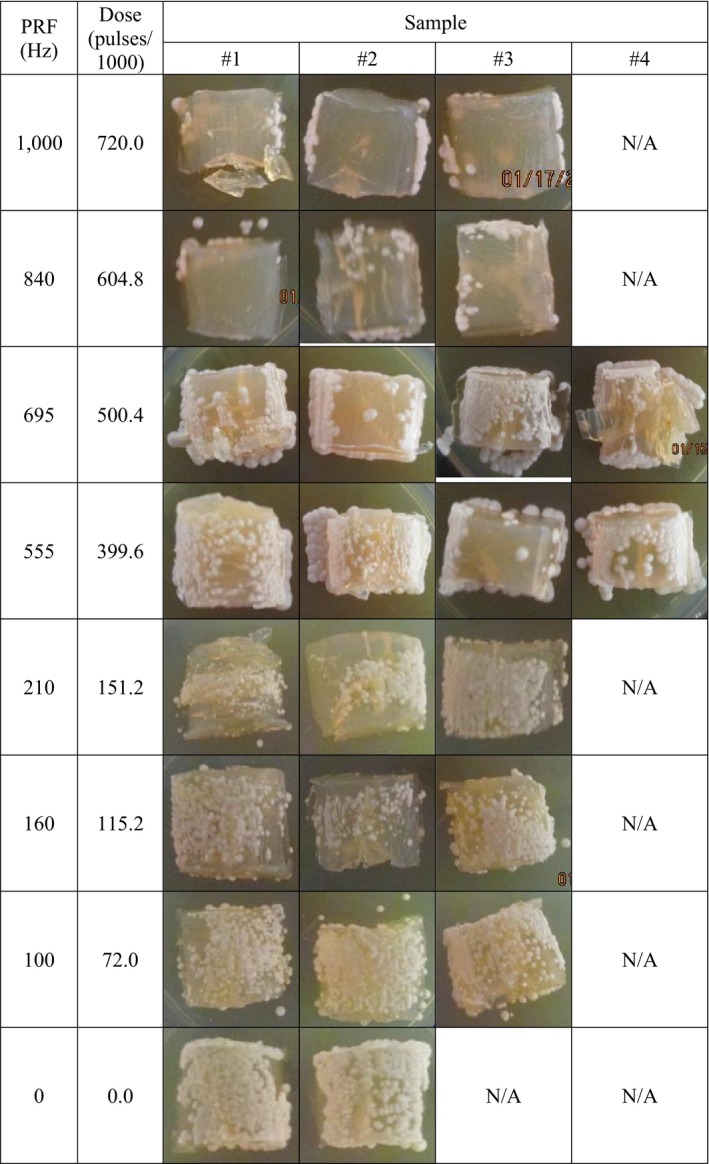
Cultured Candida albicans inoculated nail mould agar appearance after plasma treatment through the nail. Treatment conditions were AMP = 18.5 kV, SEQ = 3 × 4′(1′), and PRF as indicated. PRF = 0 Hz is untreated control. Colonies that grew under and along‐side the agar mould may have originated from cells that leaked out from under the nail before treatment and were not exposed to NTAP
A dose‐response curve was generated (Figure 5A) for the corresponding nail washes from Figure 4. The solid curve in Figure 5A is the least‐squares best fit to Equation 2 with D 50 = 63.6 kPulses, h = 1.50, and r 2 = .922. The solid curve in Figure 5B is the transform of this best‐fit equation. The dashed curves in Figure 5 are the best fit to the log‐reduction–transformed dose‐response curve with the following best‐fit parameters: D 50 = 89.0 kPulses, h = 2.53, and r 2 = 0.834. A 50% kill ratio is achieved at about 64 kPulse, 90% at about 212 kPulses, and 99% at about 550 kPulses.
4. DISCUSSION
This study has demonstrated that NTAP efficiently eliminates both C albicans and T mentagrophytes when applied directly to these organisms on agar. (“Elimination” is specifically defined as the loss of viability of fungal growth in a defined medium from washes of NTAP‐treated fungal‐infected nails, as well as on treated agar moulds.) The efficacy threshold of NTAP to kill C albicans when shielded by a nail plate has been established at doses 15 times greater than for the unshielded fungus. This efficacy is achieved for single exposures of 12 minutes or less to NTAP, as compared to the recommended protocol for efinaconazole of daily application for 48 weeks.
Although the mechanism(s) responsible for NTAP action are not fully understood, thermal tolerance measurements have shown there is insufficient thermal energy during treatment to eliminate C albicans by this mechanism alone. A temperature of at least 50°C for at least 10 minutes is required to kill C albicans; however, the “nail bed” temperature during NTAP treatment was under 50°C at efficacious dose rates. Therefore, electric field and/or reactive species, possibly in synergy with elevated temperatures, is likely the cause of the efficacy. Considering onset of pain is reached when the temperature inside the epidermis reaches 44°C20 and the temperature range for reversible thermal inactivation of tissue is from 44‐60°C,21 typical treatment temperatures of under 50°C implies that there may be some discomfort but no permanent damage to the nail bed during clinical application of NTAP.
This study employed a fairly large sample of human toe nails and statistically significant data were generated; however, these nails are not necessarily representative of nails that may be encountered clinically. Also, the population dynamics of mixed infections with different fungal isolates typical of onychomycosis encountered in clinic was not a parameter in this study. Nevertheless, we believe that NTAP is a promising treatment strategy that is cost and time effective, produces no systemic side effects, and can be modulated to suit specific nail texture and curvature. Furthermore, the design of the delivery system permits the simultaneous treatment of several infected digits and allows for controlled and repeatable follow‐up treatments, if necessary. We conclude that NTAP is an effective non‐invasive therapeutic methodology that should be evaluated in a clinical setting with or without topical agents currently in use for onychomycosis.
CONFLICTS OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
JMB, DL, ID, GF, JG, MZ, RKT conceptualised the study; JMB, DL, ID curated the data; JMB involved in formal analysis; GF, MZ, RKT involved in funding acquisition; DL, ID, SP involved in the investigation of the study; DL, ID, SP, MZ involved in methodology; JMB, JG, MZ, RKT involved in project administration; DL, SP, SS involved in resources; JMB developed software; JMB, JG, RKT involved in supervision of the study; JMB, DL, ID, JG involved in validation of the study; JMB, DL, ID involved in visualisation of the study; JMB, DL, ID, GF, JG, RKT writing – original draft; JMB, DL, ID, JG, RKT writing – review and editing.
ETHICAL APPROVAL
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to.
Bulson JM, Liveris D, Derkatch I, et al. Non‐thermal atmospheric plasma treatment of onychomycosis in an in vitro human nail model. Mycoses. 2020;63:225–232. 10.1111/myc.13030
JMB, DL, and ID should be considered joint first author.
REFERENCES
- 1. Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835‐851. [DOI] [PubMed] [Google Scholar]
- 2. Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853‐867. [DOI] [PubMed] [Google Scholar]
- 3. Ledon JA, Savas J, Franca K, Chacon A, Nouri K. Laser and light therapy for onychomycosis: a systematic review. Lasers Med Sci. 2014;29:823‐829. [DOI] [PubMed] [Google Scholar]
- 4. Fridman A, Friedman G. Plasma Medicine. New York, NY: Wiley; 2013. [Google Scholar]
- 5. Weltmann K‐D, von Woedtke TH. Plasma medicine—current state of research and medical application. Plasma Phys Control Fusion. 2017;59:014031. [Google Scholar]
- 6. Laroussi M, Alexeff I, Kang W. Biological decontamination by non‐thermal plasma. IEEE Trans Plasma Sci. 2000;28:184‐188. [Google Scholar]
- 7. Montie TC, Kelly‐Wintenberg K, Roth JR. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans Plasma Sci. 2000;28:41‐50. [Google Scholar]
- 8. Schlegel J, Koritzer J, Boxhammer V. Plasma in cancer treatment. Clin Plasma Med. 2013;1(2):2‐7. [Google Scholar]
- 9. Heinlin J, Maisch T, Zimmermann JL, et al. Contact‐free inactivation of Trichophyton rubrum and Microsporum canis by cold atmospheric plasma treatment. Future Microbiol. 2013;8:1097‐1106. [DOI] [PubMed] [Google Scholar]
- 10. Lipner SR, Friedman G, Scher RK. Pilot study to evaluate a plasma device for the treatment of onychomycosis. Clin Exp Dermatol. 2017;42:295‐298. [DOI] [PubMed] [Google Scholar]
- 11. Laroussi M. Plasma medicine: a brief introduction. Plasma. 2018;1:47‐60. [Google Scholar]
- 12. Duan J, Lu X, He G. On the penetration depth of reactive oxygen and nitrogen species generated by a plasma jet through real biological tissue. Phys Plasmas. 2017;24:073506. [Google Scholar]
- 13. He T, Liu D, Xu H, et al. A ‘tissue model’ to study the barrier effects of living tissues on the reactive species generated by surface air discharge. J Phys D Appl Phys. 2016;49:205204. [Google Scholar]
- 14. Im Y, Xiong Z, Elg DT, Graves DB. Uptake and diffusion of plasma‐generated reactive nitrogen species through keratinized membrane. J Phys D Appl Phys. 2019;52:195201. [Google Scholar]
- 15. Kogelschatz U. Dielectric‐barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma Process. 2003;23(1):1‐46. [Google Scholar]
- 16. Egot‐Lemaire S, Pijanka J, Sulé‐Suso J, Semenov S. Dielectric spectroscopy of normal and malignant human lung cells at ultra‐high frequencies. Phys Med Biol. 2009;54:2341‐2357. [DOI] [PubMed] [Google Scholar]
- 17. Gabriel C. Chapter 3 – Dielectric properties of biological materials In: Greenebaum B, Barnes FS, eds. Bioengineering and Biophysical Aspects of Electromagnetic Fields. New York, NY: Taylor & Francis; 2006:52‐94. [Google Scholar]
- 18. Cumming G, Fidler F, Vaux D. Error bars in experimental biology. J Cell Bio. 2007;177(1):7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gadagkar SR, Call GB. Computational tools for fitting the Hill equation to dose–response curves. J Pharmacol Toxicol Methods. 2015;71:68‐76. [DOI] [PubMed] [Google Scholar]
- 20. Hatton AP, Halfdanarson H. Role of contact resistance in skin burns. J Biomedical Eng. 1982;4:97‐102. [DOI] [PubMed] [Google Scholar]
- 21. Standard Guide for Heated System Surface Conditions that Produce Contact Burn Injuries. ASTM C1055‐99; 1999.


