Abstract
Aims
To assess whether people with type 2 diabetes transferring from higher basal insulin doses (> 20 units) to a starting dose of 16 units of insulin degludec/liraglutide (IDegLira) benefit from IDegLira with/without transient loss of glycaemic control.
Methods
Post hoc analysis of DUAL V and VII assessed fasting self‐measured blood glucose (SMBG) over weeks 1–8, changes in HbA1c, body weight and mean insulin dose over 26 weeks, and percentage of participants achieving HbA1c < 53 mmol/mol (7.0%) by end of trial in participants with type 2 diabetes uncontrolled with basal insulin. IDegLira was compared with continued up‐titration of insulin glargine (IGlar U100) in DUAL V, or switching to basal–bolus therapy in DUAL VII (IGlar U100 and insulin aspart), across pre‐trial insulin dose groups (20–29, 30–39 and 40–50 units/day).
Results
In all subgroups, participants treated with IDegLira experienced significant improvements in HbA1c by end of trial, which were greater than with IGlar U100 up‐titration (estimated treatment difference –5.86, –6.59 and –6.91 mmol/mol for pre‐trial insulin doses of 20–29, 30–39 and 40–50 units/day, respectively) and similar to basal–bolus therapy (estimated treatment difference –0.16, –1.0 and –0.01 mmol/mol for pre‐trial insulin doses of 20–29, 30–39 and 40–50 units/day, respectively). Compared with IGlar U100 and basal–bolus therapy, IDegLira participants experienced weight loss vs. weight gain, lower rates of hypoglycaemia and a lower mean end of trial total daily insulin dose. In both trials, mean fasting SMBG decreased from weeks 1 to 8 across all subgroups, despite a temporary increase in mean fasting SMBG in the 40–50 units pre‐trial insulin dose group during week 1 [mean increase (sd) +1.1 (2.0) mmol/l for DUAL V and +1.1 (2.1) mmol/l for DUAL VII], which reverted to baseline by week 4.
Conclusions
Regardless of pre‐trial insulin dose, IDegLira resulted in improved clinical outcomes, even in participants transferring from 40–50 units of basal insulin, despite a transient (< 4 weeks), clinically non‐relevant, elevation in pre‐breakfast SMBG. (Clinical Trial Registry Number NCT01952145 and NCT02420262).
What's new?
IDegLira is a once‐daily, fixed‐ratio insulin degludec/liraglutide combination for people with type 2 diabetes (starting dose 16 units following basal insulin or glucagon‐like peptide‐1 receptor agonists).
This post hoc analysis of DUAL V and VII demonstrated that benefits of IDegLira over basal and basal–bolus insulin are observed after switching from 20–29, 30–39 or 40–50 units of basal insulin.
People with type 2 diabetes uncontrolled on 20–39 units of basal insulin maintained glycaemic control during initial weeks when switching to IDegLira. People switching from 40–50 units of basal insulin also experienced improved clinical outcomes, but may require additional monitoring in the first four weeks.
What's new?
IDegLira is a once‐daily, fixed‐ratio insulin degludec/liraglutide combination for people with type 2 diabetes (starting dose 16 units following basal insulin or glucagon‐like peptide‐1 receptor agonists).
This post hoc analysis of DUAL V and VII demonstrated that benefits of IDegLira over basal and basal–bolus insulin are observed after switching from 20–29, 30–39 or 40–50 units of basal insulin.
People with type 2 diabetes uncontrolled on 20–39 units of basal insulin maintained glycaemic control during initial weeks when switching to IDegLira. People switching from 40–50 units of basal insulin also experienced improved clinical outcomes, but may require additional monitoring in the first four weeks.
Introduction
IDegLira is a once‐daily, titratable, fixed‐ratio combination therapy developed for the treatment of type 2 diabetes. It is composed of the basal insulin degludec U100 (degludec) and the glucagon‐like peptide‐1 receptor agonist (GLP‐1RA), liraglutide 1. The benefits of combining therapies that target different pathways are well established in several chronic disease areas 2, 3 and type 2 diabetes is no exception. Combination therapies such as GLP‐1RA/basal insulin can be more effective in lowering glycaemia in people with very high HbA1c [> 86 mmol/mol (10.0%) and/or 22 mmol/mol (2.0%) above target], in people with type 2 diabetes uncontrolled by oral anti‐diabetic drugs, or as a simple intensification option in people inadequately controlled on either basal insulin or GLP‐1RA 4, 5, 6. This approach, especially when used as a fixed‐ratio combination, has the benefit of improving control of both fasting plasma glucose (FPG) and postprandial glucose without increasing the risk of weight gain or hypoglycaemia commonly seen with addition of prandial insulin or up‐titration of basal insulin 4, 5, 6.
The DUAL clinical trial programme investigated the efficacy and safety of once‐daily IDegLira, including in people inadequately controlled on basal insulin in combination with oral anti‐diabetic drugs 7, 8, 9. Results demonstrated that IDegLira combined the clinical advantages of each component (control of FPG and postprandial glucose), while mitigating the primary side effects of treatment with basal insulin (weight gain, hypoglycaemia) and GLP‐1RA therapy (gastrointestinal adverse events). IDegLira provides a simple treatment intensification option for people requiring similar dose adjustments and blood glucose monitoring to a basal insulin therapy 10.
In DUAL V and VII, participants with type 2 diabetes inadequately controlled with 20–50 units of basal insulin were randomized to transition to IDegLira vs. continued basal insulin up‐titration (DUAL V) or basal–bolus insulin therapy (DUAL VII) 7, 8, 9. IDegLira was initiated at 16 units (16 units degludec/0.58 mg liraglutide) in both studies, according to dosing instructions 11, and titrated twice‐weekly in 2‐unit increments, based on fasting blood glucose readings. Using this starting dose, the liraglutide component was initiated close to the recommended 0.6 mg daily dose 11, 12. For participants receiving higher basal insulin doses, the initial IDegLira dosing often resulted in a reduced basal insulin dose; of note, the mean pre‐trial insulin dose in the two studies was 31–34 units daily. Although mean FPG and HbA1c levels of the overall DUAL V and DUAL VII trial populations showed improvement over the course of the trial, the question remains whether: (1) these beneficial effects are noted regardless of insulin starting dose; and (2) there may be a deterioration in glycaemic parameters soon after switching to IDegLira treatment, especially in those participants receiving higher doses of basal insulin prior to IDegLira initiation.
This post hoc analysis investigated the safety and efficacy of initiating IDegLira at 16 units vs. continued insulin glargine 100 units/ml (IGlar U100) up‐titration (DUAL V) or basal–bolus therapy (DUAL VII) when switching from various doses of basal insulin. Therefore, we assessed the fasting self‐measured blood glucose (SMBG) and other glycaemic parameters in participants across a range of pre‐trial insulin doses (20–29, 30–39 and 40–50 units/day) following treatment randomization.
Participants and methods
Study design and participants
To assess the safety and efficacy of initiating IDegLira at 16 units across the range of pre‐trial insulin doses, we performed a post hoc analysis of the two DUAL trials that enrolled participants inadequately controlled on 20–50 units of IGlar U100 in combination with metformin: DUAL V (NCT01952145) 8 and DUAL VII (NCT02420262) 9. The comparator arms were continued up‐titration of IGlar U100 (DUAL V) or basal–bolus therapy [continued up‐titration of IGlar U100 once‐daily + initiated insulin aspart (IAsp) up to four times daily, DUAL VII] 8, 9.
We compared treatment effects on participants grouped by pre‐trial insulin dose into three categories: 20–29, 30–39 and 40–50 units/day. Both trials were phase III, open‐label, two‐arm parallel, randomized, controlled, treat‐to‐target trials, with a treatment period of 26 weeks and their designs have been described previously 8, 9. Briefly, adults with uncontrolled type 2 diabetes (HbA1c 53–97 mmol/mol; 7.0–10.0%) receiving 20–50 units IGlar U100 and metformin, with a BMI ≤ 40 kg/m2, were randomized 1:1 to receive IDegLira or comparator, with pre‐trial metformin, for 26 weeks 8, 9. Prior to the initiation of both trials, the protocol, consent form, and patient information sheet were reviewed by an independent ethics committee. Both trials were conducted in accordance with International Conference on Harmonisation Good Clinical Practice 13 and the Declaration of Helsinki 14.
IDegLira was initiated at 16 units (16 units degludec/0.58 mg liraglutide) once daily, independent of meals, at approximately the same time each day and titrated to a maximum dose of 50 units (50 units degludec/1.8 mg liraglutide) 8, 9. IGlar U100 was administered once daily according to local labelling and continued at the pre‐trial dose (DUAL V), or a dose equivalent to the pre‐trial dose as part of a basal–bolus regimen (DUAL VII). There was no maximum dose of either IGlar U100 or IAsp 8, 9. Details of dose adjustments of IDegLira and IGlar U100 are provided in Doc. S1.
The following outcome measures were assessed in the two studies: change from baseline in HbA1c after 26 weeks of treatment (primary endpoint); changes in body weight and hypoglycaemic episodes (confirmatory secondary efficacy endpoints) over 26 weeks; insulin dose after 26 weeks of treatment; percentage of participants achieving HbA1c <53 mmol/mol (7.0%) (supportive secondary efficacy endpoints). The trial product was discontinued if the fasting SMBG values taken on three consecutive days, or if any of the FPG samples analysed by the central laboratory exceeded the limit of 15.0 mmol/l from baseline to week 6, 13.3 mmol/l from week 7 to week 12, 11.1 mmol/l from week 13 to week 26, or if there was no treatable intercurrent cause for the hyperglycaemia. Plots showing individual participants’ fasting SMBG over the first 8 weeks were generated to provide additional data for every participant within the subgroup that were not captured by reporting only the mean SMBG.
Criteria for withdrawal in DUAL V and rescue in DUAL VII are described in Doc. S1.
Statistical analysis
The primary analyses from the DUAL V and DUAL VII trials were repeated according to pre‐trial insulin dose subgroups of 20–29, 30–39 and 40–50 units/day. In DUAL V, continuous variables (insulin dose and change in HbA1c, FPG and body weight) were analysed using an analysis of covariance model with subgroup, treatment, region (Europe, Africa, North America, South America, Australia) and interaction between subgroup and treatment and region as fixed factors and baseline response as covariates. Data were based on the full analysis set, with missing data imputed using the pre‐planned method of last observation carried forward, as specified in the protocol. In DUAL VII, for continuous variables, a mixed model for repeated measurements with an unstructured covariance matrix was used. The model includes subgroup, treatment, visit and region as fixed factors and baseline response as covariate. The interactions between subgroup × treatment × visit, region × visit, baseline response × visit are included in the model (× denotes interaction). Data were based on the full analysis set.
In both trials, hypoglycaemia (confirmed in DUAL V; severe or blood glucose‐confirmed symptomatic in DUAL VII) was analysed using a negative binomial regression model with a log link and the logarithm of the time an event was considered treatment‐emergent as offset based on the full analysis set with subgroup, treatment, interaction between subgroup and treatment and region as fixed factors.
Results
Baseline characteristics by pre‐trial dose group
Baseline characteristics were broadly similar across pre‐trial insulin dose groups (Table S1). Body weight and BMI were slightly higher in the highest pre‐trial dose groups.
Glycaemic control
In DUAL V, participants receiving IDegLira had significantly greater HbA1c reductions from baseline to end of trial vs. IGlar U100 for all pre‐trial dose groups (P < 0.0001 for all, Fig. 1a). In DUAL VII, participants achieved similar levels of HbA1c reductions for both IDegLira and basal–bolus therapy at end of trial, regardless of pre‐trial insulin dose group (Fig. 1b). Subgroup results were in line with the overall population 8, 9. There was no significant interaction between pre‐trial insulin dose group and treatment in either the DUAL V (P = 0.85) or DUAL VII (P = 0.86) trials for change in HbA1c from baseline to end of trial; there was no effect of pre‐trial dose group on the observed differences between IDegLira and comparator.
Figure 1.
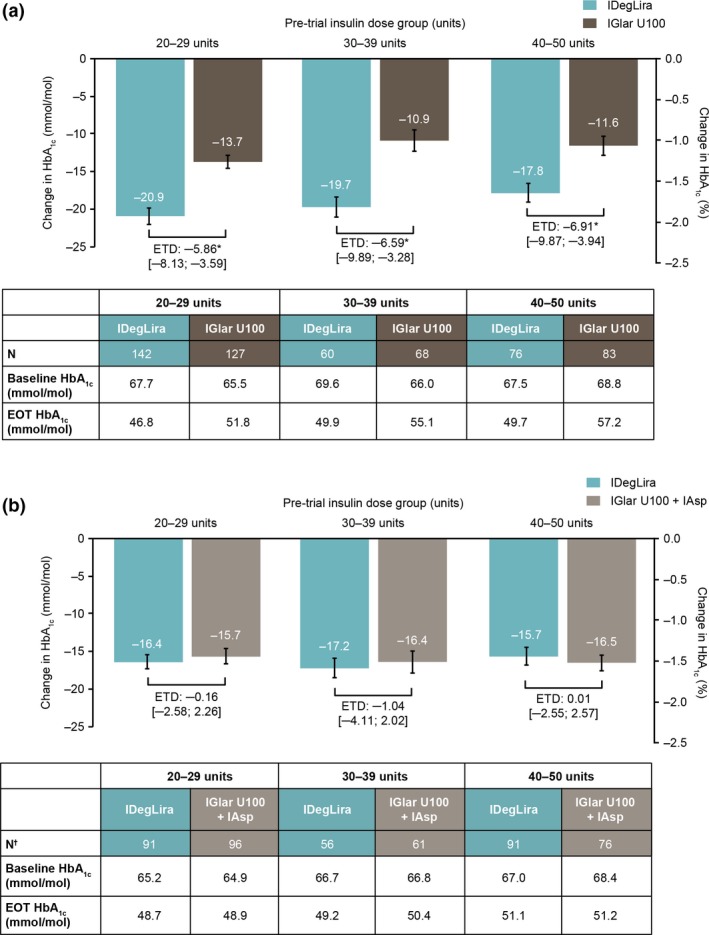
Change in HbA1c by pre‐trial insulin dose group in the (a) DUAL V and (b) DUAL VII trials. Data are observed means based on the full analysis set using either last observation carried forward imputed data for DUAL V or observed data with no imputation for DUAL VII. In DUAL V, change in HbA1c was analysed using an analysis of covariance (ANCOVA) model (including the variables: subgroup, treatment, interaction between subgroup and treatment and region as fixed factors and baseline response as covariates). In DUAL VII, the mixed model of repeated measures (MMRM) included subgroup, treatment, visit and region as fixed factors and baseline response as covariate and the interactions subgroup × treatment × visit, region × visit, baseline response × visit. Mean value (± SEM) at EOT. *P ≤ 0.0001. N† is the number of participants with a recorded absolute change in HbA1c. There was no significant interaction between pre‐trial insulin dose groups and treatment arm in either the DUAL V (P = 0.85) or DUAL VII (P = 0.86) trials in HbA1c from baseline to end of trial. EOT, end of trial; ETD, estimated treatment difference (with 95% confidence intervals); IAsp, insulin aspart; IDegLira, insulin degludec/liraglutide; IGlar U100, insulin glargine 100 units/ml.
More participants achieved HbA1c < 53 mmol/mol (7.0%) at end of trial in DUAL V with IDegLira vs. IGlar U100 in all pre‐trial dose groups (Fig. 2a). The following proportions of participants achieved HbA1c < 53 mmol/mol (7.0%) with IDegLira vs. IGlar U100: 78.2% vs. 58.3% (20–29 units group), 65.0% vs. 44.1% (30–39 units group), and 64.5% vs. 32.5% (40–50 units group). In DUAL VII, the following proportions of participants reached HbA1c < 53 mmol/mol (7.0%) at end of trial with IDegLira vs. basal–bolus therapy (Fig. 2b): 69.5% vs. 71.6% (20–29 units group), 66.7% vs. 53.8% (30–39 units group), and 52.6% vs. 55.2% (40–50 units group). Across all pre‐trial dose groups, very few participants had HbA1c > 69 mmol/mol (8.5%) at the end of the trials (Table S2). End of trial reductions in FPG were similar between treatments for all pre‐trial dose groups, in both DUAL V and DUAL VII, as expected in a treat‐to‐target trial (Fig. S1).
Figure 2.
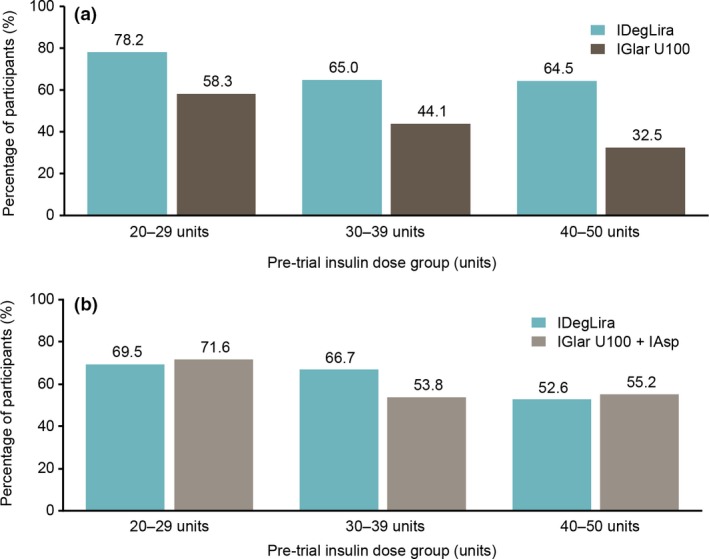
Percentage of participants reaching HbA1c < 53 mmol/mol (7.0%) at end of trial in the (a) DUAL V and (b) DUAL VII trials. Data are percentages based on the full analysis set using either observed data with last observation carried forward imputed data for DUAL V or observed data with no imputation for DUAL VII. IAsp, insulin aspart; IDegLira, insulin degludec/liraglutide; IGlar U100, insulin glargine 100 units/ml.
Mean fasting SMBG decreased between week 1 and week 8 across all pre‐trial dose groups and treatment arms for both trials (Fig. 3). In the first week post‐randomization, there was an initial increase in fasting SMBG in the 40–50 units group only, in both DUAL V and DUAL VII. In DUAL V, mean change (sd) from baseline to week 1 in fasting SMBG was –0.5 (2.1) mmol/l in the 20–29 units group, 0.3 (2.4) mmol/l in the 30–39 units group and 1.1 (2.0) mmol/l in the 40–50 units group with IDegLira. In DUAL VII, mean change (sd) from baseline to week 1 in fasting SMBG was –0.3 (1.8) mmol/l in the 20–29 units group, –0.2 (1.6) mmol/l in the 30–39 units group and 1.1 (2.1) mmol/l in the 40–50 units group with IDegLira. With the highest pre‐trial dose (40–50 units), mean fasting SMBG decreased after the first week and was at or below baseline by week 4 (Fig. 3). Between only two and four participants in the 40–50 units group had a SMBG of > 11 mmol/l at the end of the trials (Fig. S2). Plots showing individual participants’ fasting SMBG over the first 8 weeks with the highest pre‐trial dose (40–50 units) of each trial are shown in Fig. S2.
Figure 3.
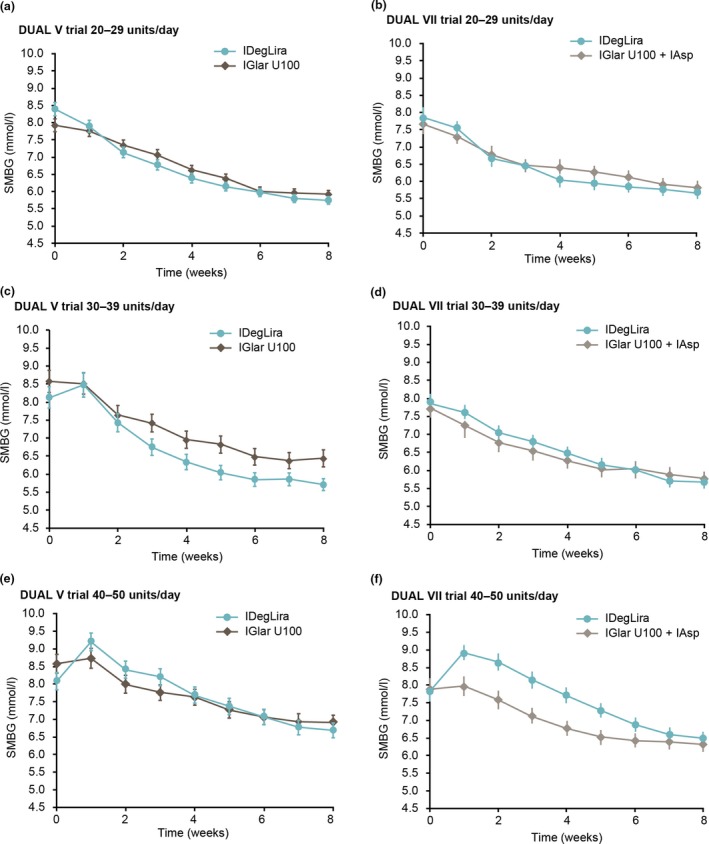
Fasting SMBG by pre‐trial insulin dose group in the first 8 weeks. Participants in the (a) DUAL V and (b) DUAL VII trials receiving 20–29 units/day; participants in the (c) DUAL V and (d) DUAL VII trials receiving 30–39 units/day; participants in the (e) DUAL V and (f) DUAL VII trials receiving 40–50 units/day. Data are based on the full analysis set using either observed data with last observation carried forward imputed data for DUAL V or observed data with no imputation for DUAL VII. IAsp, insulin aspart; IDegLira, insulin degludec/liraglutide; IGlar U100, insulin glargine 100 units/ml; SMBG, self‐measured blood glucose.
During the first 8 weeks of DUAL V, there was one withdrawal due to high SMBG levels; this occurred with IGlar U100. In DUAL VII, trial treatment was permanently discontinued in one participant in the IDegLira treatment arm due to high SMBG levels (Table S3).
Insulin dose
End of trial insulin dose was significantly lower (P < 0.0001) with IDegLira vs. comparator across all pre‐trial dose groups (Fig. 4) in both trials. End of trial insulin dose increased with increasing pre‐trial dose in all treatment arms, but to a far greater extent in the comparator arms. In DUAL V, end of trial mean insulin dose (sd) was 38.1 (11.2), 40.6 (9.6) and 46.3 (7.2) units/day with IDegLira with 20–29, 30–39 and 40–50 units/day pre‐trial insulin dose groups, respectively, whereas it was 53.3 (24.2), 68.8 (26.1) and 84.2 (31.4) units/day, respectively, with IGlar U100. The same was true of DUAL VII, with mean IDegLira dose (sd) reaching 36.0 (11.6), 40.2 (10.3) and 44.9 (7.2) units/day for the 20–29, 30–39 and 40–50 units/day pre‐trial insulin dose groups, respectively, compared with a mean basal insulin dose of 41.9 (19.9), 51.4 (20.1) and 66.1 (26.1) units/day [total daily dose 69.4 (38.6), 85.9 (41.0) and 101.2 (48.9) units/day]. As a result, the treatment difference increased with increasing pre‐trial insulin doses, resulting in a significant interaction between pre‐trial insulin dose and treatment in both the DUAL V and DUAL VII (P < 0.0001) trials for end of trial insulin dose.
Figure 4.
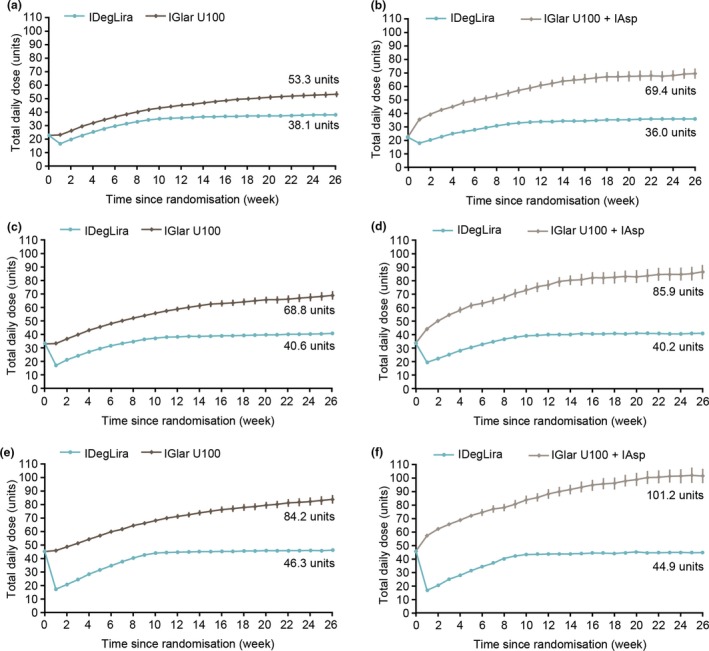
Total actual daily insulin dose over time by pre‐trial insulin dose group. Participants in the (a) DUAL V and (b) DUAL VII trials receiving 20–29 units/day; participants in the (c) DUAL V and (d) DUAL VII trials receiving 30–39 units/day; participants in the (e) DUAL V and (f) DUAL VII trials receiving 40–50 units/day. Data are observed data based on the full analysis set using either last observation carried forward imputed data for DUAL V or observed data with no imputation for DUAL VII. IAsp, insulin aspart; IDegLira, insulin degludec/liraglutide; IGlar U100, insulin glargine 100 units/ml.
Participants in comparator arms continued to up‐titrate their insulin dose throughout the study, whereas the dose of IDegLira reached a plateau at ~ 12 weeks, despite the insulin dose being reduced in the IDegLira arm rather than continued with the comparator.
Body weight
On average, IDegLira provided body weight loss vs. body weight gain with comparator across all pre‐trial dose groups in both trials, with the estimated treatment difference being significant for all comparisons (P < 0.0001) (Fig. 5). There was a significant interaction between pre‐trial insulin dose and treatment in DUAL VII (P = 0.03), but not DUAL V (P = 0.17).
Figure 5.
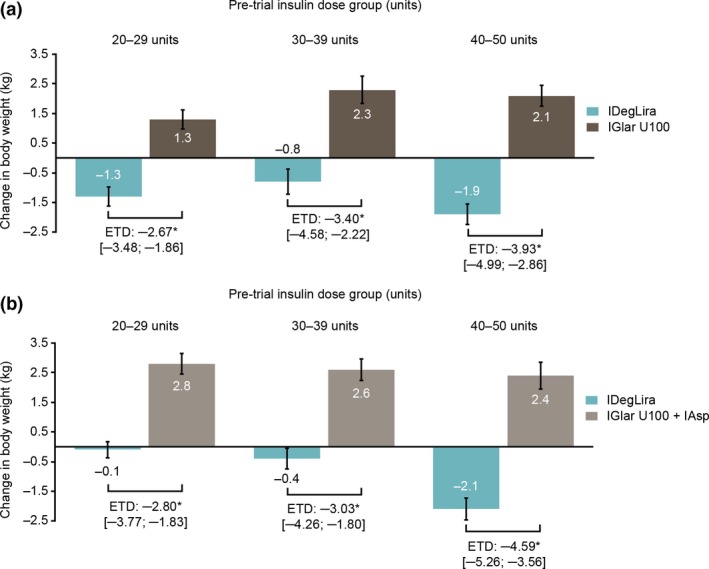
Change in body weight by pre‐trial insulin dose group in the (a) DUAL V and (b) DUAL VII trials. Data are observed means based on the full analysis set using either last observation carried forward imputed data for DUAL V or observed data with no imputation for DUAL VII. In DUAL V, change in body weight was analysed using an analysis of covariance (ANCOVA) model (including the variables: subgroup, treatment, interaction between subgroup and treatment and region as fixed factors and baseline response as covariates). In DUAL VII, the mixed model of repeated measures (MMRM) included subgroup, treatment, visit and region as fixed factors and baseline response as covariate and the interactions subgroup × treatment × visit, region × visit, baseline response × visit. *P ≤ 0.0001. There was no significant interaction between pre‐trial insulin dose groups and treatment arm in the DUAL V (P = 0.17) trial; however, there was a significant interaction in DUAL VII (P = 0.03) in participants’ body weight. ETD, ETD, estimated treatment difference; IAsp, insulin aspart; IDegLira, insulin degludec/liraglutide; IGlar U100, insulin glargine 100 units/ml.
Hypoglycaemia
In both DUAL V and DUAL VII, rates of hypoglycaemia were significantly lower in participants treated with IDegLira vs. comparator for all pre‐trial dose groups (Table 1). The greatest treatment difference in hypoglycaemic events was seen in the 40–50 units groups of each trial, with rate reductions of 85% and 77% with IDegLira vs. basal–bolus and IGlar U100, respectively. There was no significant interaction between pre‐trial insulin dose group and treatment in either the DUAL V (P = 0.09) or DUAL VII trials (P = 0.23) for hypoglycaemia rates.
Table 1.
Hypoglycaemic events by pre‐trial insulin dose group
| Hypoglycaemic events/Patient‐years of exposure | ||||
|---|---|---|---|---|
| IDegLira | Comparator | IDegLira vs. comparator (estimated rate ratio) | P‐value | |
| DUAL V: IDegLira vs. IGlar U100a | ||||
| 20–29 units/day | 2.93 | 4.71 | 0.58 (0.35, 0.93) | 0.03 |
| 30–39 units/day | 2.11 | 5.02 | 0.33 (0.15, 0.68) | 0.003 |
| 40–50 units/day | 1.05 | 5.61 | 0.23 (0.12, 0.47) | < 0.0001 |
| Test for treatment by subgroup interaction | 0.09 | |||
| DUAL VII: IDegLira vs. IGlar U100 + IAspb | ||||
| 20–29 units/day | 1.33 | 9.47 | 0.21 (0.12, 0.37) | < 0.0001 |
| 30–39 units/day | 1.48 | 6.60 | 0.33 (0.16, 0.67) | 0.002 |
| 40–50 units/day | 0.56 | 7.81 | 0.15 (0.08, 0.27) | < 0.0001 |
| Test for treatment by subgroup interaction | 0.23 | |||
Data are based on the full analysis set.
Rates of confirmed hypoglycaemia (defined as episodes in which plasma glucose was confirmed biochemically as < 3.1 mmol/l, with or without symptoms or in which the participant required assistance).
Rates of severe or blood glucose‐confirmed symptomatic hypoglycaemia (defined as severe according to American Diabetes Association classification or blood glucose‐confirmed by blood glucose value < 3.1 mmol/l with symptoms consistent with hypoglycaemia).
Hypoglycaemia was analysed using a negative binomial regression model with a log link and the logarithm of the time an event was considered treatment‐emergent as offset based on full analysis set with subgroup, treatment, interaction between subgroup and treatment and region as fixed factors.
IAsp, insulin aspart; IDegLira, insulin degludec/liraglutide; IGlar U100, insulin glargine 100 units/ml.
Discussion
In summary, the present findings demonstrate that there is no loss of glycaemic control when converting from any dose between 20 and 39 units of IGlar U100 to the starting dose of 16 units IDegLira. For participants on 40–50 units of basal insulin at baseline, there is a short‐lived, small rise in fasting SMBG within the first week after switching from basal insulin to IDegLira. Fasting SMBG corrected to baseline values by week 4 of converting to IDegLira. Regardless of the pre‐trial insulin dose group, IDegLira provided statistically significantly greater HbA1c reductions compared with IGlar U100 up‐titration and similar HbA1c reductions vs. basal–bolus therapy over 26 weeks of follow‐up. This was consistent with the overall trial results and demonstrated that after 26 weeks, participants in the higher pre‐trial dose groups also benefited from improved glycaemic control with IDegLira.
Fasting SMBG with the highest pre‐trial insulin dose (40–50 units) increased in the first week after the change in treatment but improved thereafter with adequate dose up‐titration and was comparable with or below the baseline value by week 4 of the studies. To minimize the risk of short‐term deterioration in glycaemic control, we recommend that people who switch from 40–50 units of basal insulin are adequately informed of this possibility, are given parameters for which to contact their healthcare provider (fasting SMBG >15 mmol/l, as in the clinical trial protocol), and ensure twice‐weekly titration. Although untested, providers could choose to lower the original basal insulin dose instead of discontinuing it, and continue decreasing the insulin over 2–3 weeks as the IDegLira dose is increased. This strategy would seldom be needed, as significant glycaemic deterioration (requiring withdrawal or protocol‐driven rescue therapy) did not occur in any IDegLira‐treated participants of DUAL V and only one in an IDegLira‐treated participant of DUAL VII. Indeed, the individual SMBG plots showed that most participants receiving the highest pre‐trial dose of either treatment did not reach 15.0 mmol/l, and most of those who did returned to pre‐trial levels in ≤ 4 weeks.
Across all pre‐trial insulin dose groups, IDegLira treatment also provided weight loss vs. weight gain, fewer hypoglycaemic episodes and a lower end of trial insulin dose, compared with both IGlar U100 and basal–bolus therapy. These benefits are consistent with previous findings 15 and are likely attributable to the contribution of the liraglutide component 10, as well as the long duration and predictable pharmacodynamic profile of degludec 16. Liraglutide has a glucose‐dependent mechanism of action that targets postprandial excursions as well as FPG, and has been associated with decreased appetite 17. As a result, combining liraglutide with basal insulin has the benefit of improving glycaemic control with a lower risk of hypoglycaemia and weight gain 18 in an insulin‐sparing manner. These benefits appear to be more pronounced in people switching from the highest pre‐trial insulin dose groups of either trial; the largest treatment difference in end of trial insulin dose is observed in this group. This underscores the value of combining advanced treatment options, such as insulin degludec and liraglutide, in a convenient and simple treatment regimen in one pen, with once‐daily administration at any time of day independent of meals, to mitigate side effects, while also maximizing the benefits on efficacy and safety profile 19.
The outcomes described were achieved with timely titration as recommended in the prescribing information 11, 12, and this is crucial to achieving optimal outcomes with IDegLira or any insulin product. IDegLira was titrated twice‐weekly, based on the mean of three consecutive daily fasting SMBG values, to a fasting glucose target of 4.0–5.0 mmol/l. Specifically, with the highest pre‐trial dose (40–50 units), the average dose of IDegLira was ~ 40 units by week 12 and plateaued thereafter. This is in contrast to the end‐of‐study IDegLira dose reported from the EXTRA study, a European, multicentre, retrospective chart review of 611 participants with type 2 diabetes, in which participants had a mean IDegLira dose of 30.2 units at 6 months and mean HbA1c was 59 mmol/mol (7.5%), from a baseline value of 69 mmol/mol (8.5%) 20. These results suggest that real‐world outcomes could be further improved with greater focus on titration. It may be useful to inform people that the average IDegLira dose was ~ 40 units by week 12 as a frame of reference, to help empower them to titrate in a timely manner.
One limitation inherent to clinical trials is the difficulty of generalizing findings to clinical practice. This is owing to the more careful follow‐up and attention to medication adherence that people receive in the clinical trial setting. Participants in clinical trials, who are selected using strict inclusion and exclusion criteria, tend to have fewer co‐morbidities and are more motivated than the general population with type 2 diabetes, who may have more complex needs 21. However, the broad inclusion criteria in terms of BMI, insulin dose range and HbA1c ensured an overall population reflecting people with type 2 diabetes requiring intensification of their basal insulin treatment. Additionally, the comparison with basal–bolus therapy, the current gold standard of treatment intensification when basal insulin replacement is insufficient, gives important clinical information on IDegLira as a treatment option. In clinical trials, titration guidelines are more aggressively enforced than is usually done in routine clinical practice; however, any limitations regarding inadequate titration would apply equally to IDegLira and comparator, and hence conclusions based on clinical trial comparisons are expected to be valid in a broader context.
In conclusion, this post hoc analysis demonstrated that participants switching from ≤ 50 units of basal insulin to 16 units IDegLira, experienced comparable reductions in HbA1c vs. basal–bolus therapy and statistically significantly greater reductions with IDegLira vs. IGlar U100. In addition to glycaemic control, participants receiving IDegLira also experienced fewer hypoglycaemic episodes, weight loss vs. weight gain and were receiving a lower total insulin dose after 26 weeks compared with participants receiving IGlar U100 or basal–bolus therapy. Although a brief deterioration in glycaemic control was observed in some of those who switched from the highest pre‐trial insulin dose (40–50 units), this deterioration was seldom clinically relevant and was transient (lasting < 4 weeks), with timely titration.
Funding sources
This study was supported by Novo Nordisk.
Competing interests
LM has received consulting fees or honoraria from Novo Nordisk, Sanofi Aventis and Applied Therapeutics. AD has received advisory board fees from Pfizer Inc. DG has received research support from Novo Nordisk, Lilly, Boehringer‐Ingelheim, Medtronic, Johnson & Johnson and Sanofi. TV has received personal fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Sun Pharmaceuticals and grants (to her institution) from Boehringer‐Ingelheim, Eli Lilly and Novo Nordisk. KB and PO are employees of Novo Nordisk. MFR was an employee of and owned stocks in Novo Nordisk at the time of the study. IL has received research funds, consulting fees and other support from Novo Nordisk through a contract with the University of Texas Southwestern Medical Center and has received consulting fees and/or other support from Lilly, Boehringer‐Ingelheim, Sanofi, Astra Zeneca, Mannkind, Valeritas, TARGETPharma and Intarcia.
Supporting information
Doc. S1. Supplementary methods.
Figure S1. Change in FPG by pre‐trial insulin dose group in the DUAL V and DUAL VII trials.
Figure S2. Individual fasting SMBG levels of participants converting from a pre‐trial insulin dose of 40–50 units/day to being treated with IDegLira and IGlar U100 in the DUAL V trial, and IDegLira and basal–bolus therapy in the DUAL VII trial.
Table S1. Baseline characteristics by pre‐trial insulin dose group.
Table S2. Proportion of participants with HbA1c >8.5% at end‐of‐trial by pre‐trial dose group.
Table S3. Premature discontinuation of trial drug due to continuously high SMBG within the first 8 weeks.
Acknowledgements
The authors are grateful to the people who participated in this study, to Randi Grøn, Novo Nordisk, for review of and input to the manuscript, and to Kerry Guest and Catherine Jones, Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc., for writing and editing assistance, supported by Novo Nordisk.
Parts of this study were presented as a poster presentation at the American Diabetes Association, 77th Annual Scientific Sessions, 9–13 June 2017, San Diego, CA, USA.
Diabet. Med. 37, 267–276 (2020)
References
- 1. Gough SC, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P et al Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014; 2: 885–893. [DOI] [PubMed] [Google Scholar]
- 2. Taddei S. Combination therapy in hypertension: what are the best options according to clinical pharmacology principles and controlled clinical trial evidence? Am J Cardiovasc Drugs 2015; 15: 185–194. [DOI] [PubMed] [Google Scholar]
- 3. Dale J, Alcorn N, Capell H, Madhok R. Combination therapy for rheumatoid arthritis: methotrexate and sulfasalazine together or with other DMARDs. Nat Clin Pract Rheumatol 2007; 3: 450. [DOI] [PubMed] [Google Scholar]
- 4. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G et al Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association . 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018; 41: S73–S85. [DOI] [PubMed] [Google Scholar]
- 6. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA et al Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2018 executive summary. Endocr Pract 2018; 24: 91–120. [DOI] [PubMed] [Google Scholar]
- 7. Buse JB, Vilsbøll T, Thurman J, Blevins TC, Langbakke IH, Bøttcher SG et al Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014; 37: 2926–2933. [DOI] [PubMed] [Google Scholar]
- 8. Lingvay I, Pérez Manghi F, Garcia‐Hernandez P, Norwood P, Lehmann L, Tarp‐Johansen MJ et al Effect of insulin glargine up‐titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: The DUAL V randomized clinical trial. JAMA 2016; 315: 898–907. [DOI] [PubMed] [Google Scholar]
- 9. Billings LK, Doshi A, Gouet D, Oviedo A, Rodbard HW, Tentolouris N et al Efficacy and safety of IDegLira versus basal–bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care 2018; 41: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 10. Hughes E. IDegLira: Redefining insulin optimisation using a single injection in patients with type 2 diabetes. Prim Care Diabetes 2016; 10: 202–209. [DOI] [PubMed] [Google Scholar]
- 11. Novo Nordisk . Xultophy® summary of product characteristics. Bagsvaerd, Denmark: Novo Nordisk, 2018. [Google Scholar]
- 12. Novo Nordisk . Xultophy® Prescribing Information (PI). Bagsvaerd, Denmark: Novo Nordisk, 2016. [Google Scholar]
- 13. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) . ICH Harmonised Tripartite Guideline: Good Clinical Practice. ICH, 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 29 July 2019. [Google Scholar]
- 14. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 15. Lingvay I, Norwood P, Begtrup K, Langbakke IH, Perez Manghi FC. Patients with T2D treated with insulin degludec/liraglutide (IDegLira) have a greater chance of reaching glycemic targets without hypoglycemia and weight gain than with insulin glargine (IG). Diabetes 2016; 65(Suppl. 1): A63(Abstract 239–OR). [Google Scholar]
- 16. Heise T, Hovelmann U, Nosek L, Hermanski L, Bottcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol 2015; 11: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 17. Halawi H, Khemani D, Eckert D, O'Neill J, Kadouh H, Grothe K et al Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo‐controlled pilot trial. Lancet Gastroenterol Hepatol 2017; 2: 890–899. [DOI] [PubMed] [Google Scholar]
- 18. Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon‐like peptide‐1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes 2017; 10: 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vedtofte L, Knop FK, Vilsboll T. Efficacy and safety of fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Opin Drug Saf 2017; 16: 387–396. [DOI] [PubMed] [Google Scholar]
- 20. Price H, Bluher M, Prager R, Phan TM, Thorsted BL, Schultes B. Use and effectiveness of a fixed‐ratio combination of insulin degludec/liraglutide (IDegLira) in a real‐world population with type 2 diabetes: results from a European, multicentre, retrospective chart review study. Diabetes Obes Metab 2018; 20: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pratley RE. The efficacy and effectiveness of drugs for diabetes: how do clinical trials and the real world compare? Diabetologia 2014; 57: 1273–1275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Doc. S1. Supplementary methods.
Figure S1. Change in FPG by pre‐trial insulin dose group in the DUAL V and DUAL VII trials.
Figure S2. Individual fasting SMBG levels of participants converting from a pre‐trial insulin dose of 40–50 units/day to being treated with IDegLira and IGlar U100 in the DUAL V trial, and IDegLira and basal–bolus therapy in the DUAL VII trial.
Table S1. Baseline characteristics by pre‐trial insulin dose group.
Table S2. Proportion of participants with HbA1c >8.5% at end‐of‐trial by pre‐trial dose group.
Table S3. Premature discontinuation of trial drug due to continuously high SMBG within the first 8 weeks.


