Abstract
Background
Educational attainment and verbal intelligence, which indirectly reflect an individual's cognitive reserve (CR), is suggested to buffer the effect of late‐life brain degradation on cognitive performance outcome. We aimed to explore how the relationship between whole grey matter volume (GMV) and episodic memory function is altered by CR proxy as well as age in healthy older adults.
Methods
Elderly Verbal Learning Test (EVLT) and structural magnetic resonance imaging were administered to 110 community‐residing older adults. Moderated moderation model tested whether the association between whole GMV and episodic memory was moderated by both CR and chronological age.
Results
The results showed that the moderating effect of CR on Immediate Recall, Short‐delay Recall, and Recognition scores of EVLT differed across age groups. The elderly with higher CR showed steeper GMV effect on EVLT at the Age‐Younger condition, while such moderating effect was reversed in the Age‐Older condition, suggesting an alleviated brain atrophy effect in higher CR elderly.
Conclusion
These findings suggest that although higher CR elderly may exhibit earlier GMV‐related memory decline, the buffering effect of CR on the cognitive decline due to brain atrophy would become more evident in old‐old elderly people who are likely to have accumulated more neuropathological changes. This study underscores chronological age as an important moderating factor in examining the moderating role of CR in late‐life memory function.
Keywords: brain volume, cognitive aging, education, episodic memory, moderation
INTRODUCTION
Older adults without neurological conditions tend to have moderate levels of neuropathological changes, which could have a significant impact on late‐life cognitive functioning.1 However, according to the cognitive reserve (CR) hypothesis, individuals show variable cognitive outcomes even with a similar amount of brain pathology.2 Numerous epidemiological studies have demonstrated that lifetime experiences including education,3 occupational attainment,4 literacy,5 engagement in leisure,6 and social networks7 can explain the discrepancy between late‐life neuropathology and cognitive functioning outcome, couched most famously in terms of CR. For years, education and verbal intelligence have been widely used as proxies of CR,8, 9 since direct measurement of an individual's CR is elusive.
While educational attainment has extensively shown as a protective effect in delaying the onset of Alzheimer's disease,10 a growing debate on whether CR plays a moderating role in cognitive trajectories of non‐demented older adults has not been conclusive. For example, several studies showed that education does not change the rate of cognitive decline,11, 12, 13, 14 while other studies reported somewhat conflicting results showing an attenuated or steeper cognitive decline in highly educated elderly individuals.15, 16
In order to understand the neural mechanism underlying late‐life cognitive decline, grey matter volume (GMV) reduction has been utilised to gauge individual differences in brain aging.17, 18 However, most of the age‐related neuroanatomical measures do not sufficiently explain age‐related cognitive decline, possibly due to moderating factors between the brain and cognition.19 If individual differences in GMV reflect the amount of accumulated pathological changes, their influence on memory function may differ depending on an individual's cognitive resources or reserve. For example, based on the CR hypothesis, one can posit that an elderly individual with high CR may show a weaker relationship between GMV and cognitive functions withstanding more accumulated pathologies, and the evidence also supported this prediction, especially in the effect of Alzheimer's disease pathology.3, 20 However, previous studies of normal aging population found mixed or contradicting results in how educational attainment or proxies of CR alter the association between GMV and cognitive functioning. For example, two studies specifically showed that age‐related GMV variation was positively associated with cognitive functions and such a relationship was stronger in higher CR elderly individuals.21, 22, 23 Similar or null results were reported in other studies examining the moderating role of CR, showing a stronger association between pathological impact and cognitive functions in higher CR elderly.24, 25, 26 These studies indicate that a typical moderation model needs further refinement in explaining the role of CR in the normal aging process.
One possible explanation of these counterintuitive results may come from the PAQUID longitudinal study.27 This study observed that highly educated elderly showed signs of cognitive decline a decade earlier than those of with lower education, while maintaining a more prolonged compensatory period afterward. In contrast, low‐education adults showed minimal signs of cognitive decline at an earlier phase but failed to delay severe cognitive degeneration later on. A similar explanatory model suggests that progression of brain pathology leads to the non‐linear pattern of initial decline and prolonged maintenance of memory function.28, 29 That is, elderly with larger reserves can exhibit an earlier response to benign brain atrophy starting from a higher baseline, while resisting severe forms of cognitive decline in the progressed phase of brain pathology. In this regard, one possible factor which has not been considered in examining the moderating role of CR is a relative phase in aging stage. The amount of accumulated neuropathological burden will differ from relatively younger to older elderly individuals, and the active role of CR will also differ as evidenced in the PAQUID study. It is possible that while CR does not mask the earliest impact of brain atrophy, its buffering role may be active only when a significant amount of neuropathological burden is accumulated at a relatively older phase of aging. Since previous studies investigating the moderating role of CR were conducted only within a limited range of a relatively young older population, examining the differential moderating role of both CR and chronological age across a wider range of elderly populations will be important in clarifying the inconsistencies found in previous studies.
In examining the moderating role of CR, we focused on the relationship between episodic memory function and whole‐brain GMV for the following reasons. Episodic memory function is crucial in maintaining successful daily functioning across both normal and pathological aging populations,30 and subtle signs of neuropathology well correspond to the declining pattern of memory function.31 Thus, a relatively large number of studies have examined the benefiting role of CR and lifestyle factors on this specific cognitive domain.9 Moreover, accumulating evidence suggests that volumetric neural substrate of late‐life episodic memory function is widely distributed across the cortices other than medial temporal lobe structure.32, 33 Therefore, it is possible to expect that GMV variation across a whole brain adequately assesses not only the neuropathology of a specific type of dementia but also other heterogeneous age‐related neuropathological changes.
This study aimed to examine how the relationship between GMV and verbal episodic memory is altered by both CR and chronological age. By testing the moderated moderation models, we examined whether CR alters the relationship between GMV and memory function and how such moderating effect differs across age. The conditional effect of GMV at each value of moderators (CR and age) was also estimated to inspect specific patterns of three‐way interaction models. We predicted that the higher CR elderly would show stronger GMV‐memory association in young‐old conditions as the previous study indicated, whereas weaker association would be observed in old‐old conditions possibility due to the prolonged compensatory mechanism. We tested our moderation model in the episodic memory test scores obtained from a verbal learning test, the Elderly Verbal Learning Test.
METHOD
Participants
Participants were recruited from community centres or village networks who were participating either in the Cognitive Reserve in Aging Study (CRAS) or the Korean Social Life, Health and Aging Project (KSHAP), respectively. Participants were ruled out based on the following health screening exclusion criteria34 psychiatric or neurological disorders, vision or hearing problems, possessing metals in the body that cannot be removed, hypertension or diabetes uncontrollable by drugs or insulin, history of losing consciousness due to head trauma, infarction, or stroke history. To rule out the possible effects of preclinical stages in neurocognitive disorders, a screening procedure involving interviews, neuropsychological assessment, and a neuroimaging protocol was administered.
Screening and neuropsychological tests were used to further examine recent functional changes. In the CRAS dataset, those who scored significantly low in the Korean Dementia Rating Scale (K‐DRS‐2)35 were further examined from a closest associates’ interview. Similarly, in the KSHAP study, participants who scored below 1.5 standard deviations in the Mini Mental Status Examination for Dementia Screening (MMSE‐DS) or two index scores in Elderly Memory disorder Scale (EMS)36 were further examined with the interview of a close informant. The semi‐structured interview was conducted to determine whether daily functioning had significantly declined in the past year. After magnetic resonance imaging (MRI) acquisition, participants who showed visible neurological anomaly (e.g., large infarction, lesion, and head trauma) or poor image quality in the T1‐weighted image were excluded (n = 4). The data from the CRAS (n = 42) and those from the KSHAP (n = 68) were pooled and a total of 110 participants with a mean age of 72.91 years (SD = 6.38,) and mean education of 6.77 years (SD = 3.90, range = 0–20) were finalised as the dataset (Table 1). More detailed study procedures are noted in the previous literature.37, 38 The study was approved by the Institutional Review Board of Seoul National University and Yonsei University. All participants provided written informed consent for the research procedures.
Table 1.
Descriptive statistics across categorised education groups
| ≤5 years | 6–8 years | 9–11 years | ≥12 years | |
|---|---|---|---|---|
| n | 33 | 40 | 16 | 21 |
| Age† | 74.03 (59–84) | 71.55 (61–80) | 71.31 (63–80) | 70.76 (61–83) |
| Vocabulary† | 9.36 (6–16) | 16.63 (5–36) | 20.75 (7–43) | 31.9 (15–48) |
| Gender‡ | 32:1 | 29:11 | 9:7 | 14:7 |
Mean values and ranges are noted in the parentheses.
Female : male.
MRI acquisition and processing
MRIs were acquired using a 3‐Tesla MAGNETOM Trio 32 channel coil. Whole‐brain T1‐weighted images were reconstructed from 224 sagittal slices of 1 mm thickness using an MPRAGE sequence with the following parameters: repetition time = 2.3 s, echo time = 2.3 ms, field of view = 256 × 256 mm2, and flip angle = 9°. Image preprocessing was carried out using tools implemented in Statistical Parametric Mapping software (SPM12; Wellcome Department of Imaging Neuroscience, London, UK) and executed in MATLAB Version r2015b (MathWorks, http://www.mathworks.com). New segmentation algorithm implemented in SPM12 was used to classify brain tissues.39 Total intracranial volume (ICV) was calculated as the sum of total volumes of each segmented images of grey matter (GM), white matter, and cerebrospinal fluid (CSF). ICV was used as a covariate of no interest to adjust for the baseline effect of global brain volume in the regression models. Whole GMV was used to produce interaction terms with CR and age.
Neuropsychological assessment
Elderly Verbal Learning Test (EVLT) from the Elderly Memory disorder Scale (EMS)36 was used to assess episodic memory function of the older adults. EVLT is a nine‐word verbal learning test developed for low‐education elderly Koreans adopting the California Verbal Learning Test paradigm.40 EVLT consisted of nine words of three semantic categories: households, animals, and fruits. EVLT is comprised of five serially administered trials of Immediate Recalls, the Short‐delay Recall, the Long‐delay Recall, and the Recognition subtests. The number of correctly recalled or recognised words was scored. Long‐delay Recall and Recognition subtests were administered 15–30 min after the Short‐delay Recall. In previous studies, EVLT performances not only showed positive correlations with hippocampal volumes but also successfully discriminated healthy older adults from Alzheimer's disease patients.41, 42
CR was defined as the composite score comprising years of formal education and K‐WAIS‐IV Vocabulary subtest score.43 A composite score was created by summing z‐transformed measures. Education and Vocabulary have been used to gauge lifelong intellectual experiences and premorbid intelligence44 and the composite score has been widely used as a proxy measure of CR.8, 9, 45 The pairwise correlation between two measures yielded highly convergent correlation (r = 0.727, P < 0.001).
Statistical analysis
All statistical analyses were conducted using SPSS 23 (IBM, Armonk, NY, USA). Preliminary analysis was carried out to examine the overall correlations among variables of interest. Partial correlation analyses were conducted to examine whether GMV additionally explains episodic memory function when adjusted for age, education, and ICV.
We used PROCESS macro 2.16 implemented in SPSS46 to test multiple regression models predicting each subtest score of EVLT. The moderated moderation analysis model tested how the effect of GMV (X) on EVLT (Y) is moderated by CR (M), and this moderation effect (XM) is additionally moderated by age (W). The multiple regression models included two‐way interaction terms (XM, XW, MW), and a three‐way interaction term (XMW). This model examined the significance of three‐way interaction term testing whether the moderating effect of CR on GMV effect was different across age conditions. Total intracranial volume and gender effect were adjusted as covariates of no interest.
Since dividing groups with arbitrary cut‐offs can produce spurious moderation results,47 we used the PROCESS macro to automatically generate mean‐centred interaction terms and calculate the point estimate of the conditional effect at a given set of condition values, for example ‐1 SD, mean, +1 SD of the moderators. The three conditions of each CR and age were introduced to the equations. The conditional effect of GMV (X) on EVLT performances (Y) was calculated with the following equation: b 1 + b 4 M + b 5 W + b 7 MW. This equation indicates that the slope of predictor GMV is determined by the effect of CR (b 4 M), age (b 5 W), and the interaction between CR and age (b 7 MW) in addition to the main effect of GMV (b 1). Likewise, the conditional effect of GMV × CR (XM) on EVLT across age (W) conditions were calculated with the following equation: b 4 + b 7 W. The significantly positive coefficient of the interaction term (GMV × CR) indicates that the higher CR is associated with steeper GM‐related memory decline, whereas negative interaction effect indicates the opposite. All statistical significance was set at P < 0.05, and two‐sided tests were applied.
RESULTS
The descriptive statistics and pairwise correlation coefficients of demographic and episodic memory functions were analysed (Table 2). EVLT performances were significantly associated with higher age and lower Vocabulary subtest score. Partial correlation analysis showed that GMV is positively correlated with three of the episodic memory performances when adjusting for all demographic factors and ICV (Table 3). Multiple regression models including only the two‐way interaction term (GMV × CR) did not show significant effects except for the Recognition score (P = 0.031). The three‐way interaction term (GMV × CR × Age) was significant in three of the four EVLT performances (Table 4).
Table 2.
Descriptive statistics and correlations among demographic, cognitive reserve proxies, and episodic memory function
| Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 72.11 (6.34) | 1 | 1 | |||||||
| Gender (Male : Female)† | 26:86 | 2 | 0.015 | 1 | ||||||
| Education | 6.77 (3.90) | 3 | −0.191* | 0.333** | 1 | |||||
| K‐WAIS‐IV Vocabulary | 17.96 (10.81) | 4 | −0.334** | 0.121 | 0.727** | 1 | ||||
| Immediate Recall | 28.92 (4.99) | 5 | −0.181 | −0.291** | 0.220* | 0.387** | 1 | |||
| Short‐delay Recall | 5.82(1.59) | 6 | −0.195* | −0.193* | 0.109 | 0.249** | 0.564** | 1 | ||
| Long‐delay Recall | 5.80 (1.86) | 7 | −0.333** | −0.200* | 0.044 | 0.303** | 0.515** | 0.643** | 1 | |
| Recognition | 26.53 (2.89) | 8 | −0.282** | −0.168 | 0.203* | 0.301** | 0.438** | 0.544** | 0.570** | 1 |
Spearman's correlation coefficients are noted.
P < 0.05.
P < 0.01.
Table 3.
Partial correlation between grey matter volume and Elderly Verbal Learning Test performances
| Immediate Recall | Short‐delay Recall | Long‐delay Recall | Recognition | |||||
|---|---|---|---|---|---|---|---|---|
| r | P‐value | r | P‐value | r | P‐value | r | P‐value | |
| Grey matter volume | 0.121 | 0.217 | 0.243 | 0.012 | 0.236 | 0.015 | 0.253 | 0.009 |
Age, gender, education, and intracranial volume effects were adjusted.
Table 4.
Multiple regression models predicting episodic memory performances with interaction terms
| Immediate Recall | Short‐delay Recall | Long‐delay Recall | Recognition | |||||
|---|---|---|---|---|---|---|---|---|
| B (SE) | P‐value | B (SE) | P‐value | B (SE) | P‐value | B (SE) | P‐value | |
| Age | 0.02 (0.02) | 0.314 | 0.02 (0.02) | 0.375 | −0.01 (0.02) | 0.433 | 0.01 (0.02) | 0.694 |
| GMV | 2.71 (2.23) | 0.226 | 6.14 (2.47) | 0.015 | 6.02 (2.36) | 0.012 | 5.06 (2.32) | 0.032 |
| CR | 0.32 (0.10) | 0.001 | 0.18 (0.11) | 0.103 | 0.17 (0.10) | 0.109 | 0.19 (0.10) | 0.067 |
| GMV × CR | −0.58 (1.55) | 0.711 | 0.20 (1.72) | 0.907 | 1.36 (1.65) | 0.410 | −1.57 (1.62) | 0.335 |
| GMV × age | 0.14 (0.27) | 0.614 | 0.10 (0.30) | 0.731 | 0.12 (0.29) | 0.687 | 0.47 (0.28) | 0.099 |
| CR × Age | −0.02 (0.01) | 0.147 | −0.01 (0.02) | 0.444 | −0.01 (0.02) | 0.516 | 0.01 (0.02) | 0.546 |
| GMV × CR × age | −0.75 (0.25) | 0.003 | −0.57 (0.28) | 0.042 | −0.27 (0.26) | 0.309 | −0.75 (0.26) | 0.005 |
All episodic memory scores are standardised in the models. Gender and intracranial volume effects were adjusted. GMV, grey matter volume; CR, cognitive reserve index. Statistically significant (p < 0.05) associations are indicated in bold.
In order to examine how the moderation effect of CR differs across age, the conditional GMV effect on EVLT scores was plotted at each value point of the moderator: older age condition (+1 SD = 79.2), mean age condition (mean = 72.9), and younger age condition (‐1 SD = 66.5) (Table 5). The results showed that negative moderation effect was significant at high age condition, while the opposite tendency was observed in the low age condition. In addition, nine of the point estimates of EVLT scores at values of moderating factor (CR) and GMV (−0.06, 0, 0.06) were plotted with separated age conditions (Fig. 1). At relatively younger elderly condition (−1 SD = 66.5), higher CR condition showed a steeper negative association between GMV and EVLT performances. At relatively older age condition (+1 SD = 79.2), higher CR elderly showed a mitigated association between GMV and EVLT. In contrast, lower CR elderly showed an opposite pattern, showing a steeper association between GMV and EVLT at older age condition.
Table 5.
Conditional effect of GMV × CR interactions at three age conditions
| Age condition | Immediate Recall | Short‐delay Recall | Long‐delay Recall | Recognition | ||||
|---|---|---|---|---|---|---|---|---|
| B (SE) | P‐value | B (SE) | P‐value | B (SE) | P‐value | B (SE) | P‐value | |
| Age‐older (+1 SD) | 5.34 (2.13) | 0.014 | 3.41 (2.36) | 0.152 | 0.35 (2.26) | 0.876 | 6.30 (2.22) | 0.006 |
| Age‐mean | −0.58 (1.55) | 0.711 | 0.20 (1.72) | 0.907 | 1.36 (1.65) | 0.410 | −1.57(1.62) | 0.335 |
| Age‐younger (−1 SD) | 4.18 (2.30) | 0.072 | 3.81 (2.55) | 0.138 | 3.08 (2.44) | 0.210 | 3.16 (2.39) | 0.190 |
All episodic memory scores were standardised. Gender, intracranial volume effects were adjusted. GMV, grey matter volume; CR, cognitive reserve index
Age‐older condition = 79.2; Age‐mean condition = 72.9; Age‐younger condition = 66.5. Statistically significant (p < 0.05) associations are indicated in bold.
Figure 1.
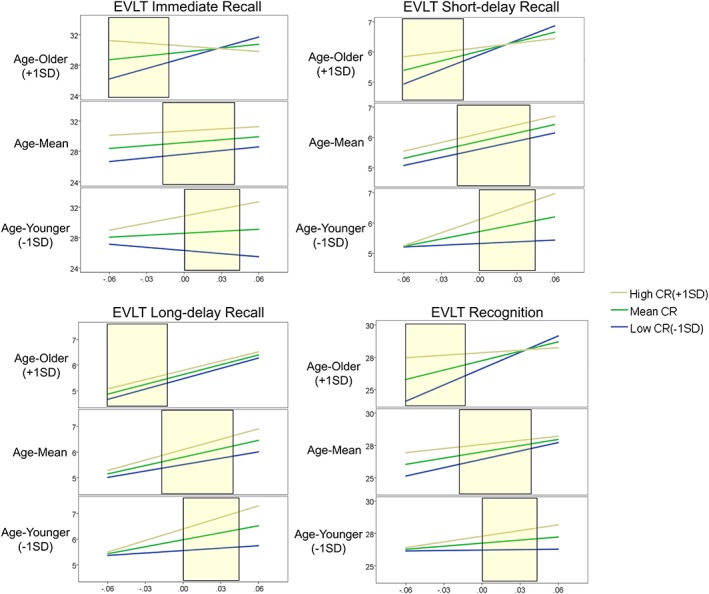
Conditional effect of grey matter volume (GMV) on Elderly Verbal Learning Test (EVLT) performances at three values of cognitive reserve index (CR), and age. The CR conditions are specified in separate lines, while age conditions are depicted in separate plots. Age and CR values at each condition points are ‐1 SD, mean, and +1 SD. Yellow windows note actual GMV value range of the trisected age groups (centre: mean GMV, width = 1 SD).
DISCUSSION
This study demonstrated how moderating effect of CR on the relationship between GMV and verbal episodic memory was further moderated by age in elderly individuals. Although CR factor alone did not moderate the relationship between GMV and episodic memory function, this moderation effect differed as a function of age conditions. The conditional effect analysis indicated that the older adults with higher CR exhibited a stronger association between GMV and memory function at a relatively younger old age, while the elderly with lower CR exhibited a stronger association between GMV and memory function at a relatively older‐old age.
A novel finding in our study is that the way CR alters the relationship between GMV and memory may gradually shift from the young‐old to old‐old period during memory aging. Although it seems counterintuitive to observe a stronger association between GMV and memory function in higher CR elderly, there are independent findings that suggest such a possibility. In previous longitudinal studies, older adults with higher education or CR showed significant cognitive decline several years earlier than the lower education group, generally starting from a higher level of cognitive functioning.15, 27, 48 As these studies have suggested, individuals with higher baseline performance may have a greater reserve to exhaust, in contrast to those with low education who may not show a decline in the initial aging process as they are likely to be already low in performance at baseline. Interestingly, while higher CR elderly exhibited earlier signs of cognitive decline, they seem to undergo a prolonged compensatory process without marked deterioration. This pattern has been observed consistently and captured in the plateau‐shaped cognitive declining model.29 This model has described a trajectory of episodic memory decline, with prolonged maintenance of cognitive functioning after an initial dip in performance.28 The earliest brain pathological change may incur a compensatory mechanism which explains rather flat trajectories afterward. When at‐risk elderly no longer can withstand the accumulated brain pathology, the steep episodic memory decline occurs in the late preclinical period. Although great cautions are required in interpreting our cross‐sectional data results, a stronger GMV‐memory association in high CR at young‐old conditions may indicate the first response to benign brain pathological burden before undergoing the flat compensatory period and may explain why previous studies showed a stronger effect of GMV on cognitive function in the high CR elderly people.21, 25
In contrast to consistent findings of education buffering against the effect of neuropathology in dementia,10, 49 previous studies have suggested benign and normal cognitive decline unmoderated by educational attainment in normal aging.11, 12, 26 However, this may be due to the limited range of age distribution mostly in the young‐old (60–64 years). Our study in contrast, had a wider distribution of ages, including a relatively older‐old population, which may have revealed the fuller spectrum of memory decline during normal cognitive aging. In other words, the role of CR as a buffering factor may become more evident when a significant amount of neuropathology accumulates in the aging brain. Our findings suggest that protective effect of education and vocabulary knowledge, as proxies of CR, may exert its role when sufficient amount of neuropathology is accumulated as in the later phase of the aging process. It seems that the protective role of educational experiences is deeply rooted as a reserve that manifests in the old‐old aging stage or clinically at‐risk state.
One notable point in the study is that the completion of basic formal education may play a crucial role in resisting and adapting to accumulated neuropathology. Literacy and arithmetic as well as basic reasoning and conceptualisation capacity taught in elementary or middle school can profoundly affect the person's CR needed in older age.50, 51, 52 Considering that a significant proportion of the elderly population, especially women, received minimal education in South Korea, our findings have important implications on the high prevalence of dementia in South Korea, and how these people may be vulnerable to late‐life neuropathological accumulation.53
There are several limitations to the current study that should be noted. First, we used the tissue segmentation method to estimate the total volume of GM and baseline brain size (ICV), but this method not only measures volume shrinkage but also reflects other types of brain structural changes including tissue intensity change.54 Further investigation of brain morphometry may be required to delineate unique effects of brain aging. Second, our analysis relied on the assumption that brain volume reduction leads to cognitive decline. Despite the limitation embedded in cross‐sectional design in the aging study,55 brain volume adjusted with intracranial volume shows moderate consistency with longitudinal brain change rate,17 suggesting that whole GMV can be used as an indirect measurement of how much an individual is undergoing brain volume changes. Lastly, the impact of neuropathological change on episodic memory function may differ across brain regions and further investigation is needed to clarify whether the moderating pattern occurs in a more regional pattern.
In conclusion, this study was able to suggest one possible reason why the protective role of CR in normal cognitive aging has been elusive. Although age and educational attainment are formally used as demographic norms to ascertain whether an individual's cognitive function has significantly declined or not, such norm‐based decision criteria and brain structural markers seem to have complications that cannot be straightforwardly adjusted with stratified norms.56 The results suggest that age and CR factors not only affect the neuropsychological function in an independent fashion but also simultaneously interact with a neuropathological marker. The meaning of the neuropathological sign in a relatively younger and higher CR elderly individual may require distinct interpretation from an older and lower CR elderly, implicating a qualitatively different at‐risk state. Future studies considering these moderating factors will be able to elucidate how older adults undergo dynamic cognitive aging processes.
ACKNOWLEDGMENT
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF‐2017S1A3A2067165). We thank Y. Cho and Y. Ha for constructive feedback. We also thank H. Kim for conducting data collection.
Disclosure: The authors have no potential conflicts of interest to disclose.
Ministry of Education of the Republic of Korea and the National Research Foundation of Korea NRF‐2017S1A3A2067165
REFERENCES
- 1. Hedden T, Schultz AP, Rieckmann A et al. Multiple brain markers are linked to age‐related variation in cognition. Cereb Cortex 2014; 26: 1388–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002; 8: 448–460. [PubMed] [Google Scholar]
- 3. Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid but not tangles with cognitive function. Neurology 2005; 65: 953–955. [DOI] [PubMed] [Google Scholar]
- 4. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. Jama 1994; 271: 1004–1010. [PubMed] [Google Scholar]
- 5. Manly JJ, Touradji P, Tang M‐X, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol 2003; 25: 680–690. [DOI] [PubMed] [Google Scholar]
- 6. Scarmeas N, Zarahn E, Anderson KE et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol 2003; 60: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 2006; 5: 406–412. [DOI] [PubMed] [Google Scholar]
- 8. Stern Y. Cognitive reserve. Neuropsychologia 2009; 47: 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Opdebeeck C, Martyr A, Clare L. Cognitive reserve and cognitive function in healthy older people: a meta‐analysis. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2016; 23: 40–60. [DOI] [PubMed] [Google Scholar]
- 10. Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology 2007; 69: 1657–1664. [DOI] [PubMed] [Google Scholar]
- 11. Zahodne LB, Glymour MM, Sparks C et al. Education does not slow cognitive decline with aging: 12‐year evidence from the Victoria longitudinal study. J Int Neuropsychol Soc 2011; 17: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tucker‐Drob EM, Johnson KE, Jones RN. The cognitive reserve hypothesis: a longitudinal examination of age‐associated declines in reasoning and processing speed. Dev Psychol 2009; 45: 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lövdén M, Rönnlund M, Wahlin A, Bäckman L, Nyberg L, Nilsson L‐G. The extent of stability and change in episodic and semantic memory in old age: demographic predictors of level and change. J Gerontol B Psychol Sci Soc Sci 2004; 59: 130–134. [DOI] [PubMed] [Google Scholar]
- 14. Wilson R, Hebert L, Scherr P, Barnes L, Mendes de Leon C, Evans D. Educational attainment and cognitive decline in old age. Neurology 2009; 72: 460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zahodne LB, Stern Y, Manly JJ. Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology 2015; 29: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans. Res Aging 2007; 29: 73–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross‐sectional and longitudinal brain volume decline in aging and AD. Neurology 2005; 64: 1032–1039. [DOI] [PubMed] [Google Scholar]
- 18. Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 2003; 60: 989–994. [DOI] [PubMed] [Google Scholar]
- 19. Salthouse TA. Neuroanatomical substrates of age‐related cognitive decline. Psychol Bull 2011; 137: 753–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rentz DM, Locascio JJ, Becker JA et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 2010; 67: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steffener J, Barulli D, Habeck C, O'Shea D, Razlighi Q, Stern Y. The role of education and verbal abilities in altering the effect of age‐related gray matter differences on cognition. PLoS One 2014; 9: e91196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Shea DM, Langer K, Woods AJ et al. Educational attainment moderates the association between hippocampal volumes and memory performances in healthy older adults. Front Aging Neurosci 2018; 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Resende E de PF, Rosen HJ, Chiang K et al. Primary school education may be sufficient to moderate a memory‐hippocampal relationship. Front Aging Neurosci 2018; 10: 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Opdebeeck C, Quinn C, Nelis SM, Clare L. Does cognitive reserve moderate the association between mood and cognition? A systematic review. Rev Clin Gerontol 2015; 25: 181–193. [Google Scholar]
- 25. O'Shea DM, Fieo RA, Hamilton JL, Zahodne LB, Manly JJ, Stern Y. Examining the association between late‐life depressive symptoms, cognitive function, and brain volumes in the context of cognitive reserve. Int J Geriatr Psychiatry 2015; 30: 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Christensen H, Batterham PJ, Mackinnon AJ, Anstey KJ, Wen W, Sachdev PS. Education, atrophy, and cognitive change in an epidemiological sample in early old age. Am J Geriatr Psychiatry 2009; 17: 218–226. [DOI] [PubMed] [Google Scholar]
- 27. Amieva H, Mokri H, Le Goff M et al. Compensatory mechanisms in higher‐educated subjects with Alzheimer's disease: a study of 20 years of cognitive decline. Brain 2014; 137: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 28. Smith GE, Pankratz VS, Negash S et al. A plateau in pre‐Alzheimer memory decline: evidence for compensatory mechanisms? Neurology 2007; 69: 133–139. [DOI] [PubMed] [Google Scholar]
- 29. Twamley EW, Ropacki SAL, Bondi MW. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease. J Int Neuropsychol Soc 2006; 12: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nyberg L, Pudas S. Successful memory aging. Annu Rev Psychol 2019; 70: 219–243. [DOI] [PubMed] [Google Scholar]
- 31. Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci 2012. May; 16: 292–305. [DOI] [PubMed] [Google Scholar]
- 32. Chang YL, Bondi MW, Fennema‐Notestine C et al. Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to Alzheimer's disease. Neuropsychologia 2010; 48: 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cacciaglia R, Molinuevo JL, Sánchez‐Benavides G et al. Episodic memory and executive functions in cognitively healthy individuals display distinct neuroanatomical correlates which are differentially modulated by aging. Hum Brain Mapp 2018; 39: 4565–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christensen KJ, Multhaup KS, Nordstrom S, Voss K. A cognitive battery for dementia: development and measurement characteristics. Psychol Assess 1991; 3: 168–174. [Google Scholar]
- 35. Chey J. Korean Dementia Rating Scale 2. Seoul: Hakjisa, 2011. [Google Scholar]
- 36. Chey J. Elderly Memory Disorder Scale. Seoul: Hakjisa, 2007. [Google Scholar]
- 37. Lee J, Park H, Chey J. Education as a protective factor moderating the effect of depression on memory impairment in elderly women. Psychiatry Investig 2018; 15: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kwak S, Joo W‐T, Youm Y, Chey J. Social brain volume is associated with in‐degree social network size among older adults. Proc Biol Sci 2018; 285: 20172708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005; 26: 839–851. [DOI] [PubMed] [Google Scholar]
- 40. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test – second edition. Adult version. Manual Test 2000.
- 41. Chey J, Lee JE, Kim MJ, Kim HY. Development and standardization of the elderly verbal learning test (EVLT). Korean J Psychol Gen 2006; 25: 141–173. [Google Scholar]
- 42. Chey J, Na DG, Tae WS, Ryoo JW, Hong SB. Medial temporal lobe volume of nondemented elderly individuals with poor cognitive functions. Neurobiol Aging 2006; 27: 1269–1279. [DOI] [PubMed] [Google Scholar]
- 43. Hwang S, Kim J, Park K, Chey J, Hong S. Korean Wechsler adult intelligence test 4th edn. Daegu: Daegu Korean Psychology Corporation, 2011. [Google Scholar]
- 44. Kim S, Lee E‐H, Hwang S‐T et al. Estimation of K‐WAIS‐IV premorbid intelligence in South Korea: development of the KPIE‐IV. Clin Neuropsychol 2015. Nov 10; 29: 19–29. [DOI] [PubMed] [Google Scholar]
- 45. Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MSV, Wright CB. Construct validity of cognitive reserve in a multiethnic cohort: the northern Manhattan study. J Int Neuropsychol Soc 2009; 15: 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hayes A. Introduction to Mediation, Moderation, and Conditional Process Analysis. New York: Guilford, 2013; 3–4. [Google Scholar]
- 47. MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods 2002; 7: 19–40. [DOI] [PubMed] [Google Scholar]
- 48. Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC. Relationship between education and age‐related cognitive decline: a review of recent research. Psychogeriatrics 2015; 15: 154–162. [DOI] [PubMed] [Google Scholar]
- 49. Mortamais M, Portet F, Brickman AM et al. Education modulates the impact of white matter lesions on the risk of mild cognitive impairment and dementia. Am J Geriatr Psychiatry 2014; 22: 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol 2005; 58: 617–622. [DOI] [PubMed] [Google Scholar]
- 51. Park H, Chey J, Lee J. Vocabulary knowledge is not a predictor of general cognitive functioning in elderly people with very low educational attainment. Dement Neurocogn Disord 2017; 16: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ardila A, Ostrosky‐Solis F, Rosselli M, Gomez C. Age‐related cognitive decline during normal aging the complex effect of education. Arch Clin Neuropsychol 2000; 15: 495–513. [PubMed] [Google Scholar]
- 53. Lopes M, Hototian S, Reis G, Elkis H, Bottino C. Systematic review of dementia prevalence 1994 to 2000. Dement Neuropsychol 1987; 2007: 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Westlye LT, Walhovd KB, Dale AM et al. Differentiating maturational and aging‐related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage 2010; 52: 172–185. [DOI] [PubMed] [Google Scholar]
- 55. Raz N, Lindenberger U. Only time will tell: cross‐sectional studies offer no solution to the age‐brain‐cognition triangle—comment on salthouse (2011). Psychol Bull 2011; 137: 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hessler J, Tucha O, Förstl H, Mösch E, Bickel H. Age‐correction of test scores reduces the validity of mild cognitive impairment in predicting progression to dementia. PLoS One 2014; 9: e106284. [DOI] [PMC free article] [PubMed] [Google Scholar]


