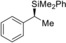
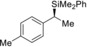
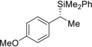
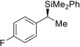
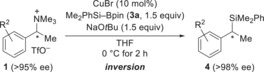
Table 2.
Stereospecific copper‐catalyzed nucleophilic substitution of benzylic ammonium triflates with a silicon nucleophile.[a]
|
Entry |
Ammonium triflate |
ee [%] |
Benzylsilane |
Yield [%][b] |
ee [%][c] |
|---|---|---|---|---|---|
|
1 |
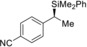
(R)‐1 a |
>99[d] |
|
79 |
>99 |
|
(S)‐4 aa | |||||
|
|
|
|
|
|
|
|
2 |
(R)‐1 b |
98[e] |
|
77 |
98 |
|
(S)‐4 ba | |||||
|
|
|
|
|
|
|
|
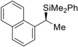
3 |
(R)‐1 c |
98[e] |
|
76 |
98 |
|
(S)‐4 ca | |||||
|
|
|
|
|
|
|
|
4 |
(S)‐1 f |
98[d] |
|
75 |
97 |
|
(R)‐4 fa | |||||
|
|
|
|
|
|
|
|
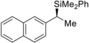
5 |
(R)‐1 g |
98[d] |
|
71 |
98 |
|
(S)‐4 ga | |||||
|
|
|
|
|
|
|
|
6 |
(S)‐1 h |
98[d] |
|
64 |
98 |
|
(R)‐4 ha | |||||
|
|
|
|
|
|
|
|
7 |
(R)‐1 j |
>95[e,f] |
|
48 |
97[g] |
|
(S)‐4 ja | |||||
|
|
|
|
|
|
|
|
8 |
(R)‐1 l |
99[d] |
|
82 |
99 |
|
(S)‐4 la | |||||
|
|
|
|
|
|
|
|
9 |
(R)‐1 m |
99[d] |
|
77 |
97 |
|
(S)‐4 ma |
[a] All reactions performed on a 0.25 mmol scale. [b] Yield of isolated product after purification by flash chromatography on silica gel. [c] Determined by HPLC analysis on chiral stationary phases after Tamao–Fleming oxidation. [d] Starting amine is commercially available in enantiomerically enriched form. [e] Determined by HPLC analysis on a chiral stationary phase at the stage of the free amine after amide formation with 4‐nitrobenzoic acid chloride (see the Supporting Information for details). [f] Racemization during amide formation; the enantiomeric excess is derived from the diastereomeric excess (de) of the highly diastereoenriched precursor10b (Ellman's tert‐butanesulfinyl amines9a). [g] The enantiomeric excess was determined at the stage of the silane; no Tamao–Fleming oxidation required.