Table 1. Nickel-catalyzed arylfluoroalkylation: optimization of conditions a .
| |||||
| Entry | Ni source | Ligand | Base | E/Z b | Yield c (%) |
| 1 d | NiCl2(PPh3)2 | XantPhos | LDA | 4 : 1 | 85 |
| 2 e | NiCl2(PPh3)2 | XantPhos | LDA | 7 : 1 | 72 |
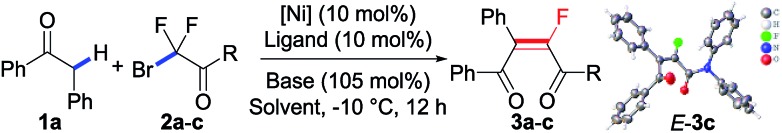
| 3 | NiCl2(PPh3)2 | XantPhos | LDA | >99 : 1 | 91 |
| 4 | NiCl2(PPh3)2 | XantPhos | tBuOK | — | Trace |
| 5 | NiCl2(PPh3)2 | XantPhos | LiHMDS | >99 : 1 | 59 |
| 6 | NiCl2(PPh3)2 | XantPhos | KHMDS | >99 : 1 | 44 |
| 7 | NiCl2 | XantPhos | LDA | >99 : 1 | 45 |
| 8 | Ni(OTf)2 | XantPhos | LDA | >99 : 1 | 14 |
| 9 | NiCl2·glyme | XantPhos | LDA | >99 : 1 | 61 |
| 10 | Ni(COD)2 | XantPhos | LDA | >99 : 1 | 85 |
| 11 | NiCl2(PPh3)2 | PPh3 | LDA | >99 : 1 | 38 |
| 12 | NiCl2(PPh3)2 | P(1-Naph)3 | LDA | >99 : 1 | 32 |
| 13 | NiCl2(PPh3)2 | dppBz | LDA | >99 : 1 | 32 |
| 14 | NiCl2(PPh3)2 | Phen | LDA | >99 : 1 | 36 |
| 15 | NiCl2(PPh3)2 | dmbPy | LDA | >99 : 1 | 20 |
| 16 | NiCl2(PPh3)2 | IPr·HCl | LDA | >99 : 1 | 18 |
| 17 f | NiCl2(PPh3)2 | XantPhos | LDA | >99 : 1 | 91 |
| 18 g | NiCl2(PPh3)2 | XantPhos | LDA | >99 : 1 | 62 |
| 19 | — | XantPhos | LDA | — | Trace |
aUnless otherwise noted, the reaction conditions were as follows: 1a (0.2 mmol), 2 (3.0 equiv.), [Ni] (10 mol%), ligand (10 mol%), base (105 mol%), solvent (2.0 mL), –10 °C, 12 h, N2.
b E/Z ratio was determined by 19F NMR analysis.
cYields of the isolated products given.
dBrCF2CO2Et was used as 2a.
eBrCF2CONEt2 was used as 2b.
f T = –30 °C.
g T = 0 °C. XantPhos = 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene, dmbPy = 4,4′-dimethyl-2,2′-bipyridine, dppBz = 1,2-bis(diphenylphosphino)benzene, Phen = 1,10-phenanthroline.
