SUMMARY
Alkali production by oral bacteria via the arginine deiminase system (ADS) increases the pH of oral biofilms and reduces the risk for development of carious lesions. This study tested the hypothesis that increased availability of arginine in the oral environment through an exogenous source enhances the ADS activity levels in saliva and dental plaque. Saliva and supra-gingival plaque samples were collected from 19 caries-free (CF) individuals (DMFT = 0) and 19 caries-active (CA) individuals (DMFT ≥ 2) before and after treatment, which comprised the use of a fluoride-free toothpaste containing 1.5% arginine, or a regular fluoride-containing toothpaste twice daily for 4 weeks. ADS activity was measured by quantification of ammonia produced from arginine by oral samples at baseline, after washout period, 4 weeks of treatment, and 2 weeks post-treatment. Higher ADS activity levels were observed in plaque samples from CF compared to those of CA individuals (P = 0.048) at baseline. The use of the arginine toothpaste significantly increased ADS activity in plaque of CA individuals (P = 0.026). The plaque microbial profiles of CA treated with the arginine toothpaste showed a shift in bacterial composition to a healthier community, more similar to that of CF individuals. Thus, an anti-caries effect may be expected from arginine-containing formulations due in large part to the enhancement of ADS activity levels and potential favorable modification to the composition of the oral microbiome.
Keywords: arginine, biofilm, plaque, microflora, caries
INTRODUCTION
Alkali production by oral bacteria influences the ecology of microbial biofilms and the chemical balance between tooth minerals by counteracting the effects of acid produced from sugar metabolism on dental plaque pH (Kleinberg, 1967b; Sissons et al., 1985, 1988; Sissons & Cutress, 1988; Dibdin & Dawes, 1998; Dawes & Dibdin, 2001). Consequently, oral alkali production has the potential to be a major endogenous caries-inhibiting factor (Burne & Marquis, 2000). In fact, considerable knowledge derived from previous clinical and laboratory studies (Stephan, 1944; Kleinberg, 1967a, 1978, 2002; Turtola & Luoma, 1972; Peterson et al., 1985; Dibdin & Shellis, 1988; Margolis et al., 1988; Sissons & Cutress, 1988; Imfeld et al., 1995; Van Wuyckhuyse et al., 1995; Dawes & Dibdin, 2001; Shu et al., 2007; Nascimento et al., 2009, 2013; Morou-Bermudez et al., 2011) supports a significant role of alkali production in oral ecology and inhibition of dental caries. For example, the metabolism of alkali-generating substrates, such as urea and arginine, by oral bacteria elicits a rise in environmental pH (Casiano-Colon & Marquis, 1988; Wijeyeweera & Kleinberg, 1989; Curran et al., 1998). Dental plaque of caries-free individuals has higher pH values and elevated ammonia levels, compared to plaque of caries-active individuals; both at rest and following a carbohydrate challenge (Stephan, 1944; Rosen & Weisenstein, 1965; Turtola & Luoma, 1972; Margolis et al., 1988). Clinical studies performed by our group revealed that increased caries risk is associated with reduced alkali-producing capacity of the microbial populations colonizing the oral cavity of adults (Shu et al., 2007; Nascimento et al., 2009), and children (Nascimento et al., 2013).
The protective principle of oral alkali production is now finding its way to the marketplace as oral care products. For example, toothpaste and mints containing arginine (Acevedo et al., 2005, 2008; Kraivaphan et al., 2013) were demonstrated to be highly effective at inhibiting the initiation and progression of dental caries, so the potential for the cost-effective use of this technology to fight caries appears high. The arginine deiminase system (ADS) is perhaps the single most significant pH homeostasis pathway in oral biofilms that influences susceptibility or resistance to dental caries. If arginolysis is to be targeted as an approach to improve oral health, additional information about the impact of delivery of exogenous arginine from oral health care products on microbial ecology is needed. This clinical study tested the hypothesis that increasing the availability of arginine in the oral environment can increase ADS activity levels in plaque and saliva of individuals with different caries experience and favorably influence the composition of the oral microbiome.
METHODS
Study group
A total of 45 adult subjects (24 females and 21 males; mean age of 26.2 ± 7.8 years) were recruited from dental clinics of the College of Dentistry at the University of Florida (UFCD). The subjects were organized into two groups consisting of 24 caries-free (CF) individuals with no clinical evidence of caries experience [decayed, missing and filled teeth (DMFT = 0)]; and 21 caries-active (CA) individuals with at least two active, cavitated and unrestored carious lesions (DT ≥ 2), independently of the number of missing and filled teeth (MFT ≥0). Of the 45 subjects who were enrolled and participated at the baseline visit, 38 (19 CF and 19 CA) complete all of the study visits. Of the seven subjects who did not complete the study, two moved away from the city, four failed to show up for one of the study visits following the baseline visit, and one was excluded from the study due to self report lack of compliance. Consequently, data was collected from 45 subjects at baseline and from 38 subjects at all other study visits. The selection process excluded subjects younger than 18 years of age and those with fewer than 20 teeth. Other exclusion criteria were systemic diseases; treatment with antibiotics, steroids or any medication known to cause dry mouth in the last 3 months; and the presence of removable or fixed dental appliances. Informed consent was obtained from each volunteer under a protocol approved by the University of Florida Health Science Center Institutional Review Board. Participating subjects completed an enrollment questionnaire describing their demographic characteristics, medical history, oral and dietary habits. Dental plaque and calculus indices were recorded visually as previously described (Greene & Vermillion, 1960). The salivary flow rate, resting pH and buffering capacity were measured during the baseline collection visit using the Saliva-check buffer kit (GC America, IL).
Study design
This single blind, randomized controlled trial had a total duration of 7 weeks and included a washout period of 1 week prior to the treatment phase, a treatment phase of 4 weeks, and a post-treatment phase of 2 weeks. During the washout period, subjects were instructed to brush their teeth twice daily for 1 min using the fluoride-containing toothpaste, Crest Cavity Protection®, for 1 week. Subjects were randomly assigned to one of the two treatment groups, which consisted of as follows: (Arg) a fluoride-free toothpaste containing 1.5% arginine, and (F) Crest Cavity Protection® (1100 ppm F as NaF). During the treatment phase, subjects were instructed to brush their teeth twice daily for 1 min using their assigned toothpaste for 4 weeks. The recommended amount of toothpaste applied to the toothbrush was shown to subjects as being about 1 g, or 2 cm in length. During the post-treatment phase, subjects discontinued the use of their assigned treatment products and continued to use whatever oral hygiene products and methods they typically used prior to the study.
Sample collection
Subjects were asked to refrain from brushing and flossing their teeth, eating, and drinking anything other than water for 12 h prior to each collection visit. Dental plaque and saliva samples were collected at time zero (baseline), after washout period (washout), after the 4 weeks of treatment (treatment), and 2 weeks after end of treatment (post-treatment). Whole unstimulated saliva was collected by asking the subjects to expectorate into a chilled sterile plastic tube. After saliva collection, supragingival plaque was pooled from all smooth surfaces of the dentition using sterile periodontal curettes. Each portion of plaque scraped from tooth surfaces was immediately transferred to and dispersed in sterile micro-centrifuge tubes containing 10 mm sodium phosphate buffer (pH 7.0). The samples were immediately transported on ice to the laboratory to be analyzed or, if necessary, snap-frozen and stored at −80°C until needed for analysis (Nascimento et al., 2009).
Arginine deiminase system activity levels of oral samples
The biochemical assays for determination of the arginolytic capacity of oral samples were performed following an established protocol (Nascimento et al., 2009). Briefly, ADS activity was measured by quantification of the ammonia produced from the incubation of plaque (25 μl) and saliva samples (5 μl) in a mixture containing 50 mm arginine-HCl (Sigma-Aldrich Inc., St. Louis, MO) and 0.5 mm Tris-Maleate buffer (pH 6.0) for 90 min at 37°C. The ammonia produced was detected by the Nessler′s Reagent (Sigma-Aldrich Inc.) using ammonium sulfate as the standard. Each sample was assayed in triplicate, and controls for background and interference were always included. ADS activity was normalized to protein content and defined as μmoles of ammonia liberated [minute × (mg of protein)]−1. The detection limit of the assay was 0.01 μmoles of ammonia liberated [minute × (mg of protein)]−1. Protein content was determined as described elsewhere (Nascimento et al., 2009).
Quantitative Real-Time PCR (qRT-PCR)
Real-Time PCR was used to quantify selected acid-and alkali-producing organisms in plaque samples collected at baseline and after 4 weeks of treatment. Species-specific oligonucleotides were used to enumerate Streptococcus mutans (gtfB gene), a well-known cariogenic species and one of the most acidogenic species of the MS group; and two arginolytic species, Streptococcus sanguinis (sagP gene) and Streptococcus gordonii (arcA gene) (Nascimento et al., 2009). The proportions of each species were obtained by normalization to total bacterial counts present in the same plaque sample as determined using universal primers for bacterial 16S rRNA genes (Rupf et al., 1999). These oligonucleotide primers were designed using DNA mfold (http://www.bioinfo.rpi.edu/applications/mfold/old/) and Beacon Designer 2.0 (Premier Biosoft International, Palo Alto, CA), and have been previously validated (Nascimento et al., 2009). DNA was extracted from plaque using the Ultra Clean Microbial DNA Isolation kit (MO BIO Laboratories Inc., Carlsbad, CA) as recommended by the supplier. A total of 10 ng of genomic DNA from each sample was used in every qRTPCR run. qRTPCRs were carried out and analyzed as described elsewhere (Nascimento et al., 2009). Of note, qRT-PCR does not differentiate viable cells from dead cells.
The Human Oral Microbe Identification Microarray (HOMIM)
The HOMIM analyses were performed at the Human Microbe Identification Microarray (MIM) Core facility at the Forsyth Institute (Cambridge, MA). HOMIM is a 16S rRNA-based microarray that allows for the simultaneous detection of about 300 of the most prevalent oral bacterial species, including many that have not yet been isolated and cultured in vitro (Paster et al., 2006; Preza et al., 2008, 2009; Colombo et al., 2009). Briefly, DNA was extracted from plaque samples collected at baseline and after 4 weeks of treatment using the Ultra Clean Microbial DNA Isolation kit (MO BIO Laboratories Inc.). The methods for amplification of 16S rRNA genes, labeling of PCR products and microarray hybridization were performed as detailed elsewhere (Preza et al., 2008; Colombo et al., 2009). The HOMIM microarrays contain 456 unique oligonucleotide probes printed in duplicate on an array that contains a total of 960 printed spots. Image analysis allowed the determination of the presence or absence of a particular microorganism based on specific criteria set for that individual spot. Data was processed at Forsyth Institute to generate microbial profile maps and cluster analyses. The method can detect the presence of organisms representing about 0.1% of the total sample and the limit of detection is about 104 cells.
Data analysis
To determine the sample size, power analyses were performed using proc power-SAS based on the method of anova test. The statistical power calculations were based on the data from a preliminary study. The analyses indicated that 45 subjects were required for addressing the aims of this study with a power of 92% (α = 0.05). For descriptive analysis, chi-square test or Fisher’s exact test was used to analyze the relationships between categorical variables. In addition, paired T-test was used to evaluate the difference of a continuous variable between baseline and after treatment. As each participant was repeatedly measured at different visits, repeated measures general linear model was used to examine the effects of caries groups and treatment on ADS activity levels and proportions of selected organisms, as determined by qRT-PCR. Akaike’s information criterion (AIC) was used to evaluate the model, where smaller AIC values suggest a better model. Contrast statement with the multiple comparisons procedure of the Tukey–Kramer HSD test was used to comparison of ADS and number of bacterial taxa. A P-value < 0.05 indicates that the predictor is significantly associated with the average change in the relative proportions of bacterial taxa between visits. All data management and statistical analyses were performed using SAS procedures (SAS 9.1.3; SAS Institute Inc., Cary, NC).
Microbial community profiles were generated from image files of scanned HOMIM arrays using a HOMIM online analysis tool (http://bioinformatics.forsyth.org/homim/). The detection of a particular taxon in a sample was determined by the presence of a fluorescent spot for that unique probe. A mean intensity for each taxon was calculated from the hybridization spots of the same probe and the signals were normalized and calculated as described elsewhere (Colombo et al., 2009). To determine how bacterial community composition varied across samples, total hybridization profiles obtained by HOMIM arrays were compared for each sample using correspondence analysis (CoA) in MeV 4.04 (Saeed et al., 2006). Analysis was done on the absolute intensity HOMIM data (frequency of scores from 0 to 5) and the prevalence of each taxon was computed for each subject and averaged within groups. To identify statistically significant differences between two groups at baseline (CA/CF subjects) and treatment (Arg/F), the Wilcoxon rank-sum test was used with the Benjamini-Hochberg correction and the False Discovery Rate (FDR) to account for multiple testing. To identify statistically significant differences between two groups before and after treatment (baseline/Arg in CF subjects, or baseline/Arg in CA subjects), paired T-test was used with the adjusted Bonferroni analysis and the FDR to account for multiple hypotheses. A P-value < 0.05 was considered significant. Sorensen index in estimates was used to compare similarity of the community composition between and within different caries groups. anova analysis with Tukey’s test (set to 0.05) on Sorensen indices was used to determine if the community composition was different among groups (JMP 8.0.1).
RESULTS
Baseline ADS activity levels
The ADS activity levels of plaque and saliva at baseline were not significantly correlated to subjects demographic characteristics, medical history, oral and dietary habits (P > 0.05). Plaque and saliva ADS activity at baseline were also not significantly correlated to plaque and calculus index, or to salivary flow rate, resting pH and buffering capacity. While significantly higher ADS activity levels were observed in plaque samples from CF (0.32 ± 0.28) compared to those of CA subjects (0.27 ± 0.33; P = 0.048), there were no statistically significant difference in the saliva ADS activity between the caries groups at baseline.
The effect of the arginine toothpaste on ammonia production
A high degree of variability in the rate of ammonia production from arginine was evident among subjects within the caries groups, irrespective of the treatment or time of collection. Table 1 presents the ADS activity levels of plaque and saliva collected from the caries groups at baseline and after the use of toothpaste containing either arginine (Arg) or fluoride (F). The effects of Arg and F on ammonia production via the ADS were dependent on the caries group. Although an increased plaque ADS activity was observed among CF subjects treated with either Arg or F, a statistically significant difference in the levels of ADS activity was observed only among those treated with F (P = 0.042). Among the CA subjects, only those making use of Arg had a statistically significant increase in plaque ADS activity (P = 0.026). General linear regression models with repeated measurements revealed that, under the conditions tested in this study, Arg increased ammonia production in the dental plaque of CA individuals.
Table 1.
Dental plaque and saliva ADS activity levels of caries-free and caries-active individuals before and after 4 weeks of treatment
| Caries group | Plaque ADS ± SD | Saliva ADS ± SD | ||
|---|---|---|---|---|
| Baseline | After treatment | Baseline | After treatment | |
| CF | 0.32 ± 0.28 | Arg: 0.41 ± 0.40 | 0.04 ± 0.04 | Arg: 0.03 ± 0.03 |
| F: 0.58 ± 0.34 | F: 0.04 ± 0.03 | |||
| CA | 0.27 ± 0.33 | Arg: 0.57 ± 0.51 | 0.03 ± 0.01 | Arg: 0.03 ± 0.02 |
| F: 0.24 ± 0.08 | F: 0.04 ± 0.02 | |||
Arginine Deiminase System (ADS) activity levels: μmoles of ammonia liberated min−1 (mg protein); SD, standard deviation; CF, caries-free individuals; CA, caries-active individuals; Arg, fluoride-free toothpaste containing 1.5% arginine; and F, fluoride-containing toothpaste.
There were no statistically significant differences in saliva ADS activity levels among the caries groups after the use of the test toothpastes. Also, there were no statistically significant differences in plaque and saliva ADS activity among the caries groups when the different collection visits (baseline, washout, treatment and post-treatment) were compared.
The effect of the arginine toothpaste on microbial communities
The proportions of two arginolytic species, S. sanguinis and S. gordonii, and the cariogenic species, S. mutans, were determined by qRT-PCR in plaque samples at baseline and after treatment (Supplemental Table S1). Multiple regression analyses revealed no correlation between the frequency of these specific bacterial species and the treatment used and/or the caries group.
Human Oral Microbe Identification Microarray was used to compare the plaque microbiome of the different caries groups before and after the use of Arg or F. Collectively, 263 bacterial species and clusters were detected from the plaque samples. Supplemental Fig. S1 shows HOMIM arrays of plaque samples collected from individuals of the different caries groups and Supplemental Fig. S2 illustrates the microbial profile comparison of the bacterial species and clusters detected.
Table 2 presents the number of bacterial taxa detected from plaque of CA and CF before and after the use of Arg or F. At baseline, a significantly higher average number of bacterial taxa was detected in the plaque of CA (99.7 ± 8.3) compared to those of CF (81.9 ± 8.2) subjects (P = 0.046). Of particular interest, a greater reduction in the numbers of bacterial species was observed in plaque samples of CA subjects treated with Arg compared to those treated with F.
Table 2.
Number of bacterial taxa detected from plaque before and after 4 weeks of treatment as determined by HOMIM
| Caries group | Number of species ± SD | |
|---|---|---|
| Baseline | After treatment | |
| CF | 81.9 ± 8.2 | Arg: 59.5 ± 6.5 |
| F: 58.3 ± 8.5 | ||
| CA | 99.7 ± 8.3 | Arg: 65.5 ± 6.8 |
| F: 81.6 ± 8.5 | ||
SD, standard deviation; CF, caries-free individuals; CA, caries-active individuals; Arg, fluoride-free toothpaste containing 1.5% arginine; F, fluoride-containing toothpaste.
The microbial profile of CA subjects was distinguished from that of CF subjects at baseline by the frequency of four bacterial species and/or clusters (Table 3). When the Wilcoxon rank-sum test with the Benjamini-Hochberg correction was used to account for multiple testing, no significant difference in bacteria taxa was found (adjusted P-value > 0.05). The microbial profiles of the caries groups at baseline are also presented in the Supplemental Fig. S3. The most prevalent species/clusters (>60% of all samples) were Streptococcus spp. Clusters III (OT 755/758/767/768/Q65) and II (OT 071/755/758/Q59), Fusobacterium nucleatum ss nucleatum and/or F. nucleatum animalis (OT 420/698/AE01), Haemophilus parainfluenzae (OT 718/W79), Veillonella atypica and/or V. parvula (OT 161/524/Q67), Capnocytophaga granulosa and/or sp clone BB167 (OT 325/326/AA89), Streptococcus oralis and/or sp clones C5MLM037 and EK048 (OT 064/707/F46), Leptotrichia buccalis and/or L. goodfellowii and/or Sneathia sanguinegens (OT 563/837/845/AA45), Streptococcus anginosus and/or S. intermedius (OT 543/644/Q62), Campylobacter gracilis (OT 623/Q04), and Campylobacter concisus and/or C. rectus (OT 575/748/X36). The oral taxon designations (OT) were defined and provided in the Human Oral Microbiome Database (HOMD; www.homd.org).
Table 3.
Mean frequencies and percentages of bacterial species that were significantly different in plaque of caries-free compared to caries-active subjects at baseline
| HOMIM Probe ID | n (%) CF | n (%) CA | P-value* | Adj. P-value |
|---|---|---|---|---|
| Selenomonas artemidis (OT124/AA49) | 19 (79%) | 7 (33%) | 0.0022 | 0.31 |
| Veillonella atypica (OT524/W88) | 18 (75%) | 6 (29%) | 0.0026 | 0.31 |
| Prevotella Cluster IV (OT658/693/714/782/AA44) | 11 (46%) | 1 (5%) | 0.0036 | 0.31 |
| Streptococcus sp. (OT070/071/N20/Hans H6/7A) | 16 (67%) | 6 (29%) | 0.0071 | 0.45 |
CF, caries-free subjects; CA, caries-active subjects; OT, Oral taxon designations for each species are defined and provided in the Human Oral Microbiome Database (http://www.homd.org/);
Wilcoxon rank-sum test with the Benjamini-Hochberg correction to account for multiple testing.
Table 4 shows the four bacterial species and/or clusters that were detected as being significantly different in the plaque of CA and the one bacterial species and/or clusters that was detected as being significantly different in the plaque of CF before and after treatment with Arg. Supplemental Table S2 shows the list of eighteen bacterial species and/or clusters that were significantly different in abundance in plaque of all subjects, independent of the caries group, before and after treatment with Arg.
Table 4.
Mean frequencies of bacterial species that were significantly different in plaque of caries-active and caries-free subjects before and after 4 weeks of treatment with the arginine toothpaste
| HOMIM Probe ID | Mean CA baseline ± SD | Mean CA Arg ± SD | n (%) CA baseline | n (%) CA Arg | Absolute t-value | Raw P-value | Adj. P-value |
|---|---|---|---|---|---|---|---|
| Veillonella parvula (OT161/D96) | 1.5 ± 1.2 | 0 | 11 (58%) | 0 | 5.5 | 3.66E-05 | 0.0095 |
| Fusobacterium nucleatum ss nucleatum and animalis (OT420/698/AD99) | 1.4 ± 0.9 | 0 | 12 (63%) | 0 | 6.2 | 9.13E-06 | 0.0024 |
| Prevotella Cluster IV (OT658/693/714/782/AA44) | 1.8 ± 0.6 | 0.6 ± 0.8 | 17 (90%) | 12 (63%) | 6.4 | 6.41E-06 | 0.0017 |
| Slackia exigua (OT602/AC97) | 1.3 ± 1.2 | 0 | 17 (90%) | 0 | 6.4 | 1.26E-04 | 00328 |
| HOMIM Probe ID | Mean CF baseline ± SD | Mean CF Arg ± SD | n (%) CF baseline | n (%) CF Arg | Absolute t-value | Raw P-value | Adj. P-value |
| Kingella oralis and Neisseria sp. clone BM052 (OT009_706_O86) | 2.4 ± 1.2 | 1.2 ± 1.1 | 17 (90%) | 13 (68%) | 5.9 | 1.73E-05 | 0.0045 |
CA, caries-active subjects; CF, caries-free subjects; Arg, fluoride-free toothpaste containing 1.5% arginine; OT, Oral taxon designations for each species are defined and provided in the Human Oral Microbiome Database (http://www.homd.org/); The analyses used data from the subjects who participated on the baseline and 4 weeks of treatment study visits. Paired t-test P < 0.05. Adjusted Bonferroni correction was applied to account for multiple testing. Degrees of freedom = 17; FDR < 0.01.
Correspondence analysis was also used to analyze the HOMIM data. Figs 1 and 2 show the diversity in the plaque microbial profile of the caries groups at baseline and after treatment with Arg. Overall, the microbiomes of CF subjects were more coherent, presenting a more similar community composition, compared to those of CA subjects. Although the Sorensen index analysis (anova and Tukey’s test) showed no statistically significant differences between and within groups, the correspondence analysis graphics showed a trend to a shift in the bacterial composition of plaque from CA treated with Arg to a community more similar to that of CF individuals.
Figure 1.
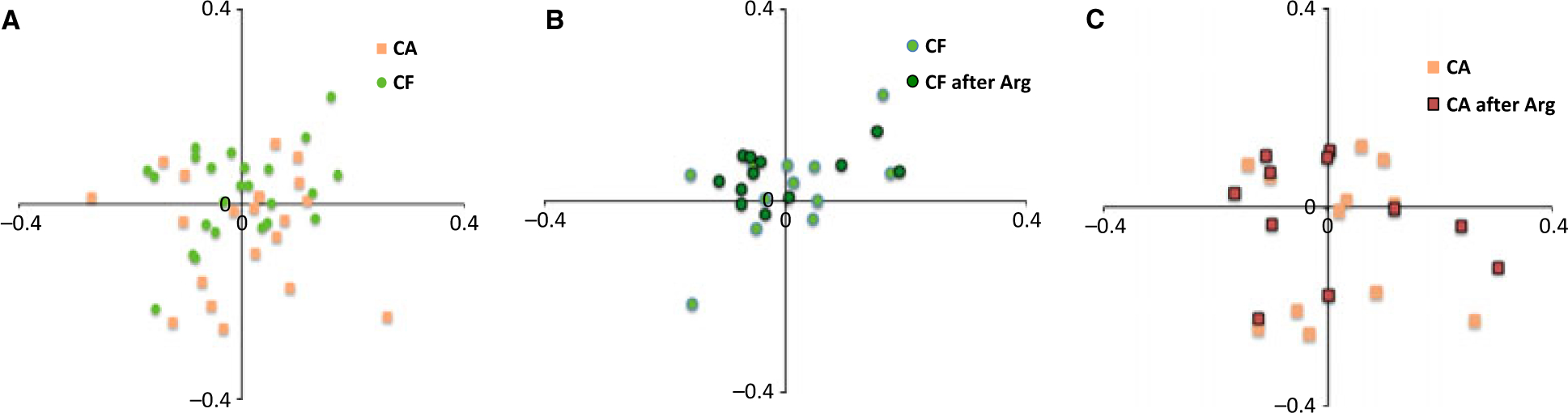
Correspondence analysis of HOMIM data at baseline and after 4 weeks of treatment with the arginine toothpaste.
Figure 2.
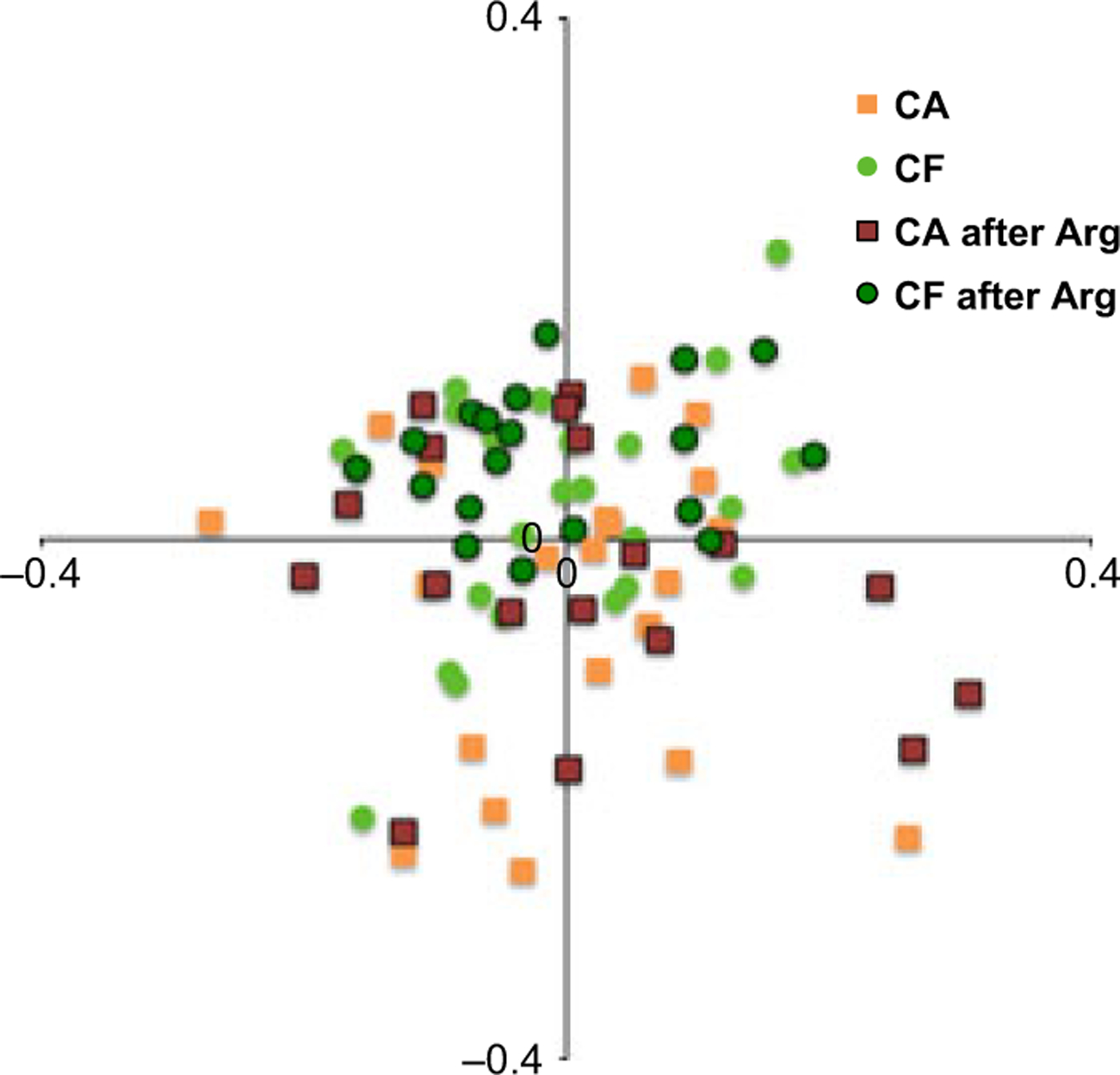
Overlaid correspondence analysis of HOMIM data showing the plaque microbiomes of the caries groups before and after treatment with Arg.
DISCUSSION
Notwithstanding the small sample size and relatively brief treatment period of this study, the results provide evidence that an increased availability of arginine in the oral environment through the use of a toothpaste containing 1.5% arginine can induce dental plaque, but not salivary, bacterial arginine deiminase system (ADS) activity. The effect was especially noted in caries-active individuals, who present a lower ADS activity at baseline compared to caries-free individuals. Therefore, an anti-caries effect may be expected from toothpaste containing arginine due, in large part, to the ability of arginine to serve as an inducer of the ADS and a substrate for ammonia production by plaque bacteria. In addition, toothpaste containing arginine may also enhance the ability of plaque bacteria to produce ammonia from naturally occurring substrates in saliva. This study supports the hypothesis that protection against caries development may be achieved by increasing the alkali-generating capacity of dental plaque (Liu et al., 2012b; Nascimento et al., 2013).
The reduction in the total number of bacterial species detected among the caries groups after treatment with the fluoride-free toothpaste containing arginine and the fluoride-containing toothpaste, al-thought not statistically significant, is likely due to an overall reduction of plaque formation associated with the introduction of a standardized oral care regimen. As previously observed (Nascimento et al., 2009), a high degree of variability in the rate of ammonia production was evident among subjects within the caries groups, irrespective of the treatment or time of collection. Our qRT-PCR results support the hypothesis that the individual oral alkalinogenic capacity may not be directly associated with a simple change in the proportions of the known ADS-positive bacterial species, S. sanguinis and S. gordonii (Nascimento et al., 2009). Instead, other bacterial species may be responsible for the bulk of oral alkali generation. Also, strain heterogeneity, differences in expression levels of the ADS genes, and/or differential inhibition of enzymatic activity may account for the high degree of variability in ammonia production in health and disease. Recently, our research group demonstrated that the microbial basis for intra-subject variations in oral arginolysis is far more complex than previously appreciated; not only may the arginolytic potential of oral biofilms be associated with the carriage of certain strains of bacteria, but also arginolytic species display a range of ADS activity as a function of environmental factors (Liu et al., 2012a). Together, these findings highlight the need for studies to characterize the bacterial species and/or microbial associations capable of contributing to total arginolysis in the oral cavity.
As determined by HOMIM, caries-active individuals presented a distinct microbial profile compared to caries-free individuals at baseline. The use of the fluoride-free toothpaste containing arginine by caries-active individuals promoted a change in plaque bacterial composition to an apparently healthier community, which is comparable to those of caries-free individuals. Here, it was demonstrated that the HOMIM technique significantly supported our goal of characterizing the microbial profile of individuals with different caries status and oral arginolytic capacities. Most importantly, the HOMIM results suggest that the modulation of the alkali-generating potential of dental plaque, perhaps by using arginine formulations, may foster an ecologically healthy oral environment that could have the potential to suppress the emergence of acid-tolerant, caries-associated pathogenic organisms. Two questions still to be answered are whether the change in the proportions of specific bacterial species and/or clusters is associated solely with the use of the fluoride-free toothpaste containing arginine and whether the identified group of species/clusters is indicative of a favorable and healthy change in the microbiome.
Much remains to be investigated with respect to the effects of novel oral care technologies containing arginine and fluoride (Acevedo et al., 2005, 2008; Kraivaphan et al., 2013) on the composition, gene expression profiles and biochemical activities of the oral microbiota. Nonetheless, based on existing knowledge of the impact of the ADS on pH homeostasis and bacterial bioenergetics, it is expected that the combined mechanisms of action of arginine and fluoride may impact the ecology of oral biofilms in a way that promotes dental health. Future studies will also continue to dissect the underlying molecular basis for the individual and caries-related differences in arginolysis. The combination of these studies will provide information about the various microbial profiles and will determine those that may be considered health- or disease-related, ultimately allowing for the identification of those individuals at greater risk for developing dental caries. Such experiments will expand the knowledge on the diversity of the oral alkali-generating bacteria and their role in oral health and disease.
Supplementary Material
Figure S1. HOMIMs of DNA isolated from dental plaque of caries-free (A) and caries-active (B) subjects at baseline.
Figure S2. (A) Bacterial profile maps of HOMIMs comparing plaque samples from different subjects. (B) Magnified from (A) illustrating dendrogram of cluster analysis and partial list of bacterial species tested. Band intensities represent relative bacterial proportions.
Figure S3. Mean frequencies of bacterial species that were detected in >40% of all plaque samples at baseline.
Table S1. Percentage of plaque bacteria from caries-free and caries-active subjects before and after 4 weeks of treatment as determined by qRT-PCR.
Table S2. Mean frequencies of bacterial species that were significantly different in plaque of all subjects before and after 4 weeks of treatment with the arginine toothpaste.
ACKNOWLEDGEMENTS
This work was supported by Colgate Palmolive. The authors declare that there are no conflicts of interest.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
REFERENCES
- Acevedo AM, Machado C, Rivera LE, Wolff M and Kleinberg I (2005) The inhibitory effect of an arginine bicarbonate/calcium carbonate CaviStat-containing dentifrice on the development of dental caries in Venezuelan school children. J Clin Dent 16: 63–70. [PubMed] [Google Scholar]
- Acevedo AM, Montero M, Rojas-Sanchez F et al. (2008) Clinical evaluation of the ability of CaviStat in a mint confection to inhibit the development of dental caries in children. J Clin Dent 19: 1–8. [PubMed] [Google Scholar]
- Burne RA and Marquis RE (2000) Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett 193: 1–6. [DOI] [PubMed] [Google Scholar]
- Casiano-Colon A and Marquis RE (1988) Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol 54: 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo AP, Boches SK, Cotton SL et al. (2009) Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol 80: 1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran TM, Ma Y, Rutherford GC and Marquis RE (1998) Turning on and turning off the arginine deiminase system in oral streptococci. Can J Microbiol 44: 1078–1085. [DOI] [PubMed] [Google Scholar]
- Dawes C and Dibdin GH (2001) Salivary concentrations of urea released from a chewing gum containing urea and how these affect the urea content of gel-stabilized plaques and their pH after exposure to sucrose. Caries Res 35: 344–353. [DOI] [PubMed] [Google Scholar]
- Dibdin GH and Dawes C (1998) A mathematical model of the influence of salivary urea on the pH of fasted dental plaque and on the changes occurring during a cariogenic challenge. Caries Res 32: 70–74. [DOI] [PubMed] [Google Scholar]
- Dibdin GH and Shellis RP (1988) Physical and biochemical studies of Streptococcus mutans sediments suggest new factors linking the cariogenicity of plaque with its extracellular polysaccharide content. J Dent Res 67: 890–895. [DOI] [PubMed] [Google Scholar]
- Greene JC and Vermillion JR (1960) The oral hygiene index: a method for classifying oral hygiene status. J Am Dent Assoc 61: 29–35. [Google Scholar]
- Imfeld T, Birkhed D and Lingstrom P (1995) Effect of urea in sugar-free chewing gums on pH recovery in human dental plaque evaluated with three different methods. Caries Res 29: 172–180. [DOI] [PubMed] [Google Scholar]
- Kleinberg I (1967a) Effect of urea concentration on human plaque pH levels in situ. Arch Oral Biol 12: 1475–1484. [DOI] [PubMed] [Google Scholar]
- Kleinberg I (1967b) Effect of varying sediment and glucose concentrations on the pH and acid production in human salivary sediment mixtures. Arch Oral Biol 12: 1457–1473. [DOI] [PubMed] [Google Scholar]
- Kleinberg I (1978) Prevention and dental caries. J PrevDent 5: 9–17. [PubMed] [Google Scholar]
- Kleinberg I (2002) A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med 13: 108–125. [DOI] [PubMed] [Google Scholar]
- Kraivaphan P, Amornchat C, Triratana T et al. (2013) Two-year caries clinical study of the efficacy of novel dentifrices containing 1.5% Arginine, an insoluble calcium compound and 1,450 ppm fluoride. Caries Res 47: 582–590. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nascimento MM, Schulte R, Kalra R and Burne RA (2012a) Characterization of the Arginolytic Microflora of Human Oral Biofilms. J Dent Res 91: 1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Nascimento MM and Burne RA (2012b) Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int J Oral Sci 4: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis HC, Duckworth JH and Moreno EC (1988) Composition of pooled resting plaque fluid from caries-free and caries-susceptible individuals. J Dent Res 67: 1468–1475. [DOI] [PubMed] [Google Scholar]
- Morou-Bermudez E, Elias-Boneta A, Billings RJ et al. (2011) Urease activity in dental plaque and saliva of children during a three-year study period and its relationship with other caries risk factors. Arch Oral Biol 56: 1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Gordan VV, Garvan CW, Browngardt CM and Burne RA (2009) Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol 24: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Liu Y, Kalra R et al. (2013) Oral arginine metabolism may decrease the risk for dental caries in children. J Dent Res 92: 604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster BJ, Olsen I, Aas JA and Dewhirst FE (2006)The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000 42: 80–87. [DOI] [PubMed] [Google Scholar]
- Peterson S, Woodhead J and Crall J (1985) Caries resistance in children with chronic renal failure: plaque pH, salivary pH, and salivary composition. Pediatr Res 19: 796–799. [DOI] [PubMed] [Google Scholar]
- Preza D, Olsen I, Aas JA, Willumsen T, Grinde B and Paster BJ (2008) Bacterial profiles of root caries in elderly patients. J Clin Microbiol 46: 2015–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preza D, Olsen I, Willumsen T et al. (2009) Microarray analysis of the microflora of root caries in elderly. Eur J Clin Microbiol Infect Dis 28: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S and Weisenstein PR (1965) The effect of sugar solutions on pH of dental plaques from caries-susceptible and caries-free individuals. J Dent Res 44: 845–849. [DOI] [PubMed] [Google Scholar]
- Rupf S, Kneist S, Merte K and Eschrich K (1999) Quantitative determination of Streptococcus mutans by using competitive polymerase chain reaction. Eur J Oral Sci 107: 75–81. [DOI] [PubMed] [Google Scholar]
- Saeed AI, Bhagabati NK, Braisted JC et al. (2006) TM4 microarray software suite. Methods Enzymol 411: 134–193. [DOI] [PubMed] [Google Scholar]
- Shu M, Morou-Bermudez E, Suarez-Perez E et al. (2007) The relationship between dental caries status and dental plaque urease activity. Oral Microbiol Immunol 22: 61–66. [DOI] [PubMed] [Google Scholar]
- Sissons CH and Cutress TW (1988) pH changes during simultaneous metabolism of urea and carbohydrate by human salivary bacteria in vitro. Arch Oral Biol 33: 579–587. [DOI] [PubMed] [Google Scholar]
- Sissons CH, Cutress TW and Pearce EI (1985) Kinetics and product stoichiometry of ureolysis by human salivary bacteria and artificial mouth plaques. Arch Oral Biol 30: 781–790. [DOI] [PubMed] [Google Scholar]
- Sissons CH, Hancock EM and Cutress TW (1988) The source of variation in ureolysis in artificial plaques cultured from human salivary bacteria. Arch Oral Biol 33: 721–726. [DOI] [PubMed] [Google Scholar]
- Stephan RM (1944) Intra-oral hydrogen-ion concentration associated with dental caries activity. J Dent Res 23: 257–266. [Google Scholar]
- Turtola LO and Luoma H (1972) Plaque pH in caries-active and inactive subjects modified by sucrose and fluoride, with and without bicarbonate-phosphate. Scand J Dent Res 80: 334–343. [DOI] [PubMed] [Google Scholar]
- Van Wuyckhuyse BC, Perinpanayagam HE, Bevacqua D et al. (1995) Association of free arginine and lysine concentrations in human parotid saliva with caries experience. J Dent Res 74: 686–690. [DOI] [PubMed] [Google Scholar]
- Wijeyeweera RL and Kleinberg I (1989) Arginolytic and ureolytic activities of pure cultures of human oral bacteria and their effects on the pH response of salivary sediment and dental plaque in vitro. Arch Oral Biol 34: 43–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. HOMIMs of DNA isolated from dental plaque of caries-free (A) and caries-active (B) subjects at baseline.
Figure S2. (A) Bacterial profile maps of HOMIMs comparing plaque samples from different subjects. (B) Magnified from (A) illustrating dendrogram of cluster analysis and partial list of bacterial species tested. Band intensities represent relative bacterial proportions.
Figure S3. Mean frequencies of bacterial species that were detected in >40% of all plaque samples at baseline.
Table S1. Percentage of plaque bacteria from caries-free and caries-active subjects before and after 4 weeks of treatment as determined by qRT-PCR.
Table S2. Mean frequencies of bacterial species that were significantly different in plaque of all subjects before and after 4 weeks of treatment with the arginine toothpaste.


