Figure 3.
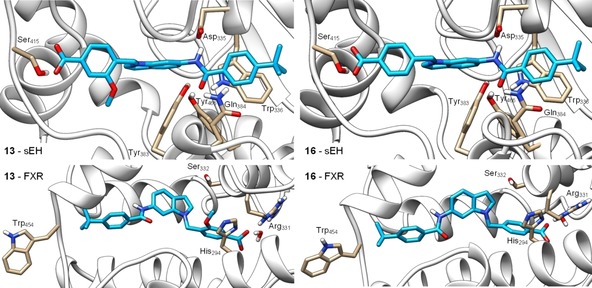
Molecular docking of 13 (left) and 16 (right) in the sEH active site (PDB‐ID: 3OTQ,23 upper images) and the ligand binding site of FXR in partially active conformation (PDB‐ID: 4QE8,24 lower images). 13 and 16 form similar predicted binding modes in the sEH active site with the amide nitrogen participating in the typical hydrogen bond to the catalytic Asp335 residue and the amide oxygen interacting with Tyr383 and Tyr466. In addition, the benzamide aromatic ring makes a stacking interaction with Trp336 and the carboxylate moiety engages a hydrogen bond with Ser415. In the FXR ligand binding site, 13 and 16 interact with Arg331 in water mediated hydrogen bonds as exclusive polar contact. The tert‐butylbenzamide moieties occupy the region of Trp454 which consequently obtains an outward conformation resulting in partial FXR activation.24 The methoxy group of 13 occupies a polar region between His294 and Ser332 which agrees with the higher potency of 13 on FXR. Docking was performed in MOE and visualized using UCSF Chimera.
