Scheme 2.
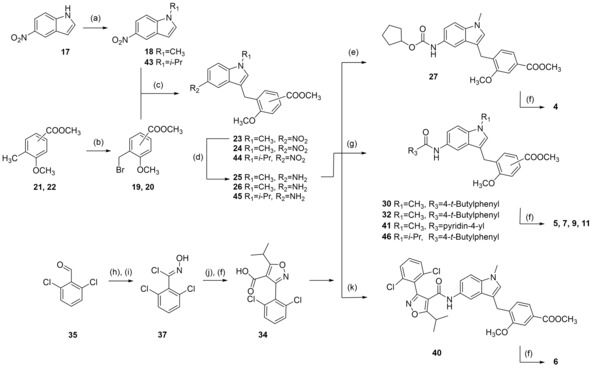
Reagents & conditions: (a) DMS or diisopropyl sulfate (42), dioxane, 40 °C, 2.5 h; (b) NBS, AIBN, CHCl3, reflux, 4 h; (c) FeCl3, dioxane, rt, 12 h; (d) H2, Pd(C), MeOH, rt, 12 h; (e) C5H9‐OCOCl (28), DIPEA, 4 °C to rt, 4 h; (f) LiOH, MeOH/H2O, rt, 16 h; (g) 4‐tert‐butylbenzoyl chloride (29), pyridine, DMF, CHCl3, reflux; or 4‐tert‐butylbenzoic acid (31) or isonicotinic acid (33), EDC*HCl, 4‐DMAP, CHCl3, reflux, 6 h; (h) H2N−OH*HCl, NaOH, EtOH, 90 °C, 24 h; (i) NCS, DMF, rt, 5 h; (j) methyl isobutyrylacetate (38), NaOMe, THF, rt, 16 h; (k) 34, EDC*HCl, 4‐DMAP, CHCl3, reflux, 6 h.
