Abstract
Objective
To evaluate the effects of tadalafil monotherapy on lower urinary tract symptoms, urodynamic parameters, and oxidative stress levels in male patients.
Methods
This prospective study included 53 male patients with urinary symptoms, who met the criteria for overactive bladder (OAB) (≥ 2 points for Q3 [urgency] in the OAB symptom score [OABSS] assessment and ≥ 3 points for the total score). The patients received 5 mg tadalafil orally once daily, and their symptoms were assessed before and after the 12‐week treatment. The OABSS and international prostate symptom score (IPSS) were used to evaluate the subjective symptoms. The objective findings were assessed using uroflowmetry. Oxidative stress was assessed by determining urinary levels of 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) levels with an adjustment for urinary creatinine (CR) concentration.
Results
After tadalafil administration, total and individual indices of the OABSS assessment showed significant improvement. In addition, total storage and voiding symptoms that contributed to the IPSS were also significantly improved. The voided volume was increased, and the maximum flow rate was improved after tadalafil treatment (P = .002 and < 0.001, respectively). Urinary 8‐OHdG/CR decreased from 12.4 ± 9.7 ng/mg CR to 7.6 ± 11.6 ng/mg CR (P < .001). In patients who showed OAB improvement and did not meet the criteria for OAB after the treatment (44 patients, 83.0%), the urinary 8‐OHdG/CR level was significantly decreased from 11.6 ± 8.4 ng/mg CR to 6.4 ± 10.3 ng/mg CR (P < .001).
Conclusions
Tadalafil treatment improves OAB symptoms and urodynamic parameters by decreasing oxidative stress level.
Keywords: lower urinary tract symptoms, overactive bladder, oxidative stress, tadalafil, urodynamic parameters
1. INTRODUCTION
Lower urinary tract symptoms (LUTS), which comprise voiding, storage, and postmicturition symptoms, are caused by various factors, including benign prostatic hyperplasia in male patients. Depending on the objective findings and patient‐subjective symptoms, drugs such as α1‐adrenergic receptor blockers, 5α‐reductase inhibitors, anticholinergic agents, β3 adrenergic receptor stimulants, or phosphodiesterase type 5 (PDE5) inhibitors are used in clinical practice. In particular, PDE5 inhibitors have been shown to ameliorate LUTS by various mechanisms, for example, by relaxing smooth muscle cells in the urogenital tract via the NO/cGMP/PDE5 pathway,1, 2 increasing bladder perfusion,3 decreasing afferent nerve activity,4 and decreasing smooth muscle proliferation.5 Hence, tadalafil, which is the only PDE5 inhibitor approved for male LUTS (mLUTS) patients, is now used worldwide.
Oxidative stress caused by bladder ischemia as a result of pelvic artery insufficiency leads to LUTS, including overactive bladder (OAB).6, 7 In addition, it has been reported that the urinary level of 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG), which is recognized as a useful oxidative stress marker, is higher in LUTS patients than in individuals without LUTS.8 Furthermore, tadalafil reduced oxidative stress both in vivo and in vitro.9, 10, 11 However, the relationship between oxidative stress level and the effectiveness of tadalafil in mLUTS patients is unclear.
Understanding molecular mechanisms of tadalafil efficacy is important for the development of a treatment strategy in patients with mLUTS. Hence, the aim of this study was to evaluate the efficacy of tadalafil monotherapy in improving urinary tract symptoms and urodynamic parameters and to clarify the relationship between such efficacy and oxidative stress changes induced by the treatment in mLUTS patients with OAB.
2. METHODS
2.1. Ethics
The study protocol was approved by the Clinical Study Review Board of the Nagasaki University Hospital. All procedures were carried out in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients that participated in the study.
2.2. Patients
mLUTS patients with OAB symptoms were recruited at the Nagasaki University Hospital (Nagasaki, Japan). This study was a prospective, single‐center, open‐label study. mLUTS patients with OAB symptoms (overactive bladder symptom score [OABSS]: urgency ≥ 2, total score ≥ 3) for at least 3 months, who did not take any medications for their urinary symptoms, were included in this study. The patients received oral tadalafil (Zalutia®; Nippon Shinyaku Co., Ltd., Kyoto, Japan; 5 mg) once daily in the morning. We evaluated the efficacy of the treatment using the OABSS, international prostate symptom score (IPSS), and visual analog scale for quality of life (QOL; rated from 0 to 10) to assess the subjective symptoms, and urinary 8‐OHdG changes 12 weeks after initial administration were compared with those at baseline. In addition, we used uroflowmetry and determined postvoid residual (PVR) urine volume to assess objective symptoms. Exclusion criteria were a PVR volume of 100 mL, prostate volume of 40 g, history of urinary retention, prior diagnosis of neurogenic bladder, urethral stricture, history of symptomatic orthostatic hypotension, history of prostatic surgery, bladder stones, renal insufficiency (glomerular filtration rate < 30 mL/min/1.73 m2), liver impairment, urological malignancy, active lower urinary tract infection, treatment with any antimuscarinic or α1 adrenal receptor antagonist agents, or conclusion about unsuitability for the trial by the treating physicians.
2.3. Urinary sample preparation and assessment
The participants were asked to come to the clinic by 10:00 am, replicating the settings described in a previous report,12 and their urine samples were collected at the maximum desire to void to measure urinary 8‐OHdG. Urine specimens taken from all subjects were centrifuged at 3000 × g for 10 min, and the supernatants were separated and stored at −80 °C until being tested. After defrosting, urinary 8‐OHdG and creatinine (CR) concentrations were measured using a human 8‐OHdG ELISA kit (New 8‐OHdG Check®; Japan Institute for the Control of Aging, Nikken Seil Co., Ltd., Shizuoka, Japan) and a creatinine assay kit (Colorimetric for Urine Sample; Cayman Chemical, Ann Arbor, Michigan), as described in the manufacturers' instructions. In addition, 8‐OHdG levels were normalized by the urinary CR concentration. Both 8‐OHdG and CR levels were determined using ELISA kits as above. The absorbance was detected at 450 nm for 8‐OHdG and at 492 nm for CR using a microplate reader (Thermo/LabSystems Multiskan RC; Artisan Technology Group, Champaign, Illinois). Forty males without any LUTS and with comorbidity background similar to that of OAB patients were selected as control subjects. After that, we evaluated the differences in urinary biomarkers between control subjects and OAB patients. In addition, we compared urinary levels of this biomarker before and after oral tadalafil treatment. Furthermore, we divided the patients into two groups by the efficacy of tadalafil treatment. In particular, we defined the patients whose urinary symptoms improved and did not fit the OAB definition after the 12‐week study period as the responder group, whereas the patients with persisting OAB symptoms after the treatment were defined as the nonresponder group. Urinary 8‐OHdG/CR levels before and after tadalafil administration were then compared between the two groups.
2.4. Statistical analysis
All statistical values are presented as the mean ± SD. The Wilcoxon signed rank test was used to evaluate changes in subjective symptoms based on the OABSS and IPSS, whereas objective findings were obtained by uroflowmetry and urinary biomarker analysis. All statistical analyses were two‐sided and conducted with a significance level of α = 0.05 (P < .05) using JMP 13 software (SAS Institute, Cary, North Carolina).
3. RESULTS
3.1. Patients' characteristics
A total of 53 mLUTS patients with OAB were enrolled in the present study (Table 1). The mean age at the start of the treatment was 72.1 ± 7.2 years. The body mass index was 23.1 ± 2.7 kg/m2, and the mean prostate volume was 24.3 ± 8.2 mL. Hypertension and renal dysfunction were frequent comorbidities in 25 (47.2%) and 22 (41.5%) patients, respectively. In addition, the levels of urinary 8‐OHdG and 8‐OHdG/CR in OAB patients were higher than those in control subjects (Figure 1).
Table 1.
Patients' characteristics at baseline
| Entire | Responders | Nonresponders | P value | |
|---|---|---|---|---|
| Number of patients (N/%) | 53 | 44 | 9 | — |
| Age (years) | 72.1 ± 7.2 | 71.3 ± 1.1 | 75.9 ± 2.3 | .071 |
| Body mass index (kg/m2) | 23.1 ± 2.7 | 23.2 ± 2.6 | 22.6 ± 3.5 | .953 |
| Prostate volume (mL) | 24.3 ± 8.2 | 24.8 ± 6.5 | 23.5 ± 5.8 | .764 |
| Comorbidity | ||||
| Hypertension (%) | 25 (47.2) | 18 (40.9) | 7 (77.8) | .067 |
| Diabetes mellitus (%) | 10 (18.9) | 10 (22.3) | 0 (0) | .181 |
| Renal dysfunction (%) | 22 (41.5) | 18 (40.9) | 4 (44.4) | 1.000 |
| Hyperlipidemia (%) | 15 (28.3) | 13 (29.6) | 2 (28.3) | 1.000 |
| Urological parameter | ||||
| VV (mL) | 116.3 ± 78.3 | 118.7 ± 84.6 | 104.3 ± 35.0 | .887 |
| Qmax (mL/s) | 7.9 ± 3.5 | 7.8 ± 3.8 | 8.5 ± 1.4 | .553 |
| PVR (mL) | 42.3 ± 31.7 | 43.2 ± 30.6 | 37.8 ± 38.5 | .129 |
| Urinary 8‐OHdG/CR (ng/mg CR) | 12.4 ± 9.7 | 11.6 ± 8.4 | 16.3 ± 14.4 | .850 |
Abbreviations: 8‐OHdG, 8‐hydroxy‐2’‐deoxyguanosine; CR, creatinine; PVR, postvoid residual; Qmax, maximum flow rate; VV, voided volume.
Figure 1.
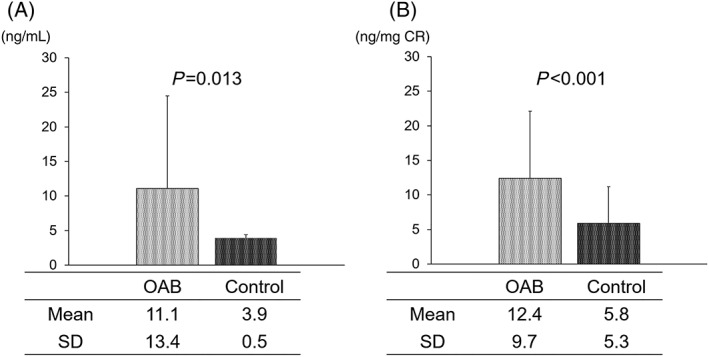
Differences in the oxidative stress marker 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) levels between overactive bladder (OAB) patients and control subjects. Absolute (A) and normalized (by creatine [CR] level) 8‐OHdG concentrations (B) levels in OAB patients were significantly higher than in control participants
3.2. Relationship between subjective and objective findings and urinary 8‐OHdG level
Table 2 shows the relationship between subjective and objective findings and urinary 8‐OHdG. Urinary 8‐OHdG levels did not correlate with subjective symptoms, except for weak stream in IPSS. However, urinary 8‐OHdG showed moderate positive correlation with voided volume (VV) and maximum flow rate (Qmax), which are objective symptoms.
Table 2.
Relationship between urinary 8‐OHdG/CR level and subjective and objective symptoms at baseline
| r | P value | |
|---|---|---|
| OABSS | ||
| Q1 (daytime frequency) | 0.1204 | .391 |
| Q2 (nighttime frequency) | 0.0164 | .907 |
| Q3 (urgency) | 0.1077 | .443 |
| Q4 (urgency incontinence) | 0.0015 | .997 |
| Total score | 0.0155 | .912 |
| IPSS | ||
| Q1 (incomplete emptying) | 0.1552 | .877 |
| Q2 (frequency) | 0.1433 | .306 |
| Q3 (intermittency) | 0.0982 | .484 |
| Q4 (urgency) | 0.1369 | .328 |
| Q5 (weak stream) | 0.3845 | .005 |
| Q6 (straining) | 0.0210 | .881 |
| Q7 (nocturia) | 0.1363 | .967 |
| Storage symptoms (Q2+Q4+Q7) | 0.0606 | .667 |
| Voiding symptoms (Q1+Q3+Q5+Q6) | 0.1770 | .204 |
| Total score | 0.1199 | .393 |
| QOL score | 0.1044 | .457 |
| Objective symptoms | ||
| VV | 0.381 | .005 |
| Qmax | 0.336 | .014 |
| PVR | 0.261 | .060 |
Abbreviations: 8‐OHdG, 8‐hydroxy‐2’‐deoxyguanosine; CR, creatinine; IPSS, international prostate symptom score; OABSS, overactive bladder symptom score; PVR, postvoid residual; Qmax, maximum flow rate; QOL, quality of life; VV, voided volume.
3.3. Changes in subjective symptoms according to OABSS and IPSS
The magnitude of subjective symptoms was determined from respective OABSS and IPSS values (Table 3). Among the OAB symptoms, tadalafil treatment improved the total score and all subscale OABSS. In particular, the total OABSS was improved from 6.5 ± 1.7 to 2.8 ± 1.9 (P < .001), and Q3 (urgency) also was decreased from 2.7 ± 0.9 to 0.9 ± 1.1 (P < .001) during the 12‐week study period. In 44 (83.0%) patients, OAB manifestations, as outlined in the Methods section, diminished with improvements in urinary symptoms after 12 weeks of tadalafil administration. In addition, the total IPSS, IPSS storage symptoms, IPSS voiding symptoms, and QOL score also improved after tadalafil treatment. All individual IPSS, except Q3 (intermittency), were significantly decreased after tadalafil treatment (Table 3).
Table 3.
Changes in the subjective symptoms
| Baseline | 12 wk | P value | |
|---|---|---|---|
| OABSS | |||
| Q1 (daytime frequency) | 0.7 ± 0.7 | 0.3 ± 0.5 | <.001 |
| Q2 (nighttime frequency) | 2.4 ± 0.7 | 1.5 ± 0.9 | <.001 |
| Q3 (urgency) | 2.7 ± 0.9 | 0.9 ± 1.1 | <.001 |
| Q4 (urgency incontinence) | 0.7 ± 0.8 | 0.1 ± 0.3 | <.001 |
| Total score | 6.5 ± 1.7 | 2.8 ± 1.9 | <.001 |
| IPSS | |||
| Q1 (incomplete emptying) | 1.6 ± 1.4 | 0.9 ± 0.7 | <.001 |
| Q2 (frequency) | 2.5 ± 1.2 | 1.2 ± 0.9 | <.001 |
| Q3 (intermittency) | 1.5 ± 1.6 | 1.2 ± 1.1 | .069 |
| Q4 (urgency) | 2.2 ± 1.3 | 0.9 ± 0.9 | <.001 |
| Q5 (weak stream) | 2.4 ± 1.6 | 1.4 ± 1.3 | <.001 |
| Q6 (straining) | 1.5 ± 1.4 | 0.9 ± 1.3 | .005 |
| Q7 (nocturia) | 2.7 ± 1.3 | 1.6 ± 1.1 | <.001 |
| Storage symptoms (Q2+Q4+Q7) | 7.3 ± 2.8 | 3.8 ± 2.4 | <.001 |
| Voiding symptoms (Q1+Q3+Q5+Q6) | 7.1 ± 4.8 | 4.0 ± 2.9 | <.001 |
| Total score | 14.4 ± 5.7 | 7.7 ± 3.4 | <.001 |
| QOL score | 4.2 ± 1.0 | 2.3 ± 0.9 | <.001 |
Abbreviations: IPSS, international prostate symptom score; OABSS, overactive bladder symptom score; QOL, quality of life.
3.4. Changes in urodynamic parameters and urinary 8‐OHdG level
Objective symptoms in the patients were assessed from the changes in urodynamic parameters (Table 4). VV and Qmax improved significantly after 12 weeks of tadalafil administration (VV: from 116.3 ± 78.3 mL to 168.4 ± 102.6 mL, P = .002; Qmax: from 7.9 ± 3.5 mL/s to 10.8 ± 5.7 mL/s, P < .001). However, no statistically significant change in PVR volume was observed (from 42.3 ± 31.7 mL to 37.3 ± 27.1 mL, P = .266). The absolute concentration of urinary 8‐OHdG, a urinary oxidative stress biomarker, decreased significantly from 11.1 ± 13.4 ng/mL to 6.0 ± 7.6 ng/mL after the 12‐week treatment period (P = .006). In addition, the level of 8‐OHdG corrected by that of urinary CR also significantly improved from 12.4 ± 9.7 ng/mg CR to 7.6 ± 11.6 ng/mg CR (P < .001; Figure 2).
Table 4.
Changes in the objective symptoms
| Baseline | 12 wk | P value | |
|---|---|---|---|
| VV (mL) | 116.3 ± 78.3 | 168.4 ± 102.6 | 0.002 |
| Qmax (mL/s) | 7.9 ± 3.5 | 10.8 ± 5.7 | < 0.001 |
| PVR (mL) | 42.3 ± 31.7 | 37.3 ± 27.1 | 0.266 |
Abbreviations: PVR, postvoid residual; Qmax, maximum flow rate; VV, voided volume.
Figure 2.
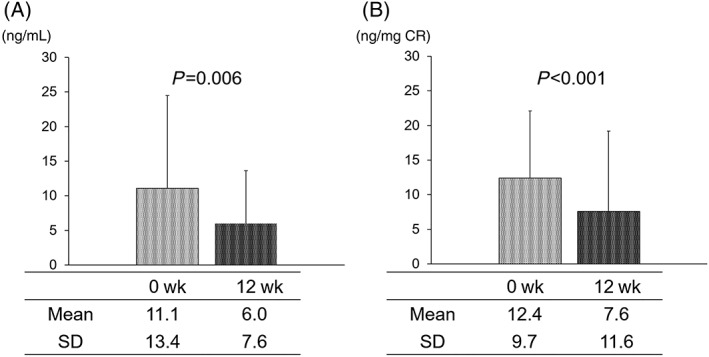
Changes in the oxidative stress marker 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) level following tadalafil treatment. Absolute (A) and normalized (by creatine [CR] level) 8‐OHdG concentrations (B) before and after 12‐week treatment. Both parameters were significantly decreased by tadalafil administration
3.5. Relationship between the improvement of OAB symptoms and 8‐OHdG level
In the responder group, which comprised 44 (83.0%) patients with improved OAB symptoms according to the OABSS, the normalized urinary 8‐OHdG level was significantly decreased from 11.6 ± 8.4 ng/mg CR to 6.4 ± 10.3 ng/mg CR post treatment (P < 0.001). However, in the nonresponder group of nine patients, (17.0%) whose OAB symptoms persisted after tadalafil treatment, the change of the urinary 8‐OHdG/CR value from 16.3 ± 14.4 to 13.6 ± 16.3 ng/mL CR was not statistically significant (Figure 3).
Figure 3.
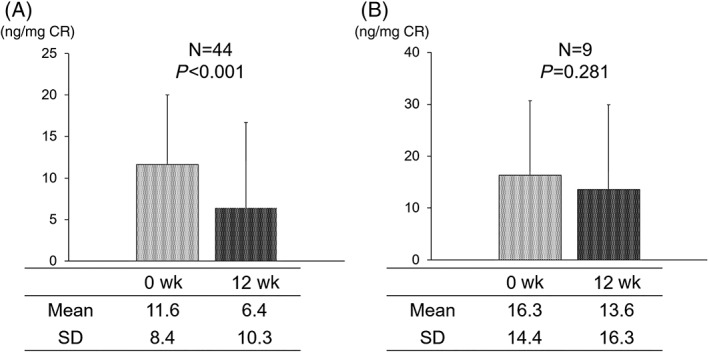
Changes in the oxidative stress marker 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) in responders and nonresponders. Urinary 8‐OHdG level was significantly decreased after 12‐week tadalafil treatment in the responder group (A) but not in the nonresponder group (B)
3.6. Adverse events
Two (3.8%) patients had epigastric distress, and one (1.9%) patient suffered from headache after taking tadalafil orally. However, all adverse events were very mild, and all participants continued their participation in this clinical study for the complete 12‐week treatment period.
4. DISCUSSION
The present study demonstrates that oral administration of tadalafil improves subjective symptoms, as assessed by the OABSS, total IPSS, and IPSS QOL, in mLUTS patients with OAB. In addition, we also found that this therapy improves urodynamic parameters such as VV and Qmax in these patients. With regard to the efficacy of tadalafil in relation to the subjective and objective symptoms, an earlier pressure flow study showed that 12‐week tadalafil treatment improved not only subjective symptoms but also the parameters such as the Qmax, detrusor overactivity, and bladder outlet obstruction index.13 However, it has also been proposed that tadalafil treatment signficantly improves symptom scores without signficantly affecting uroflowmetric measures such as Qmax, PVR volume, maximum detrusor pressure, and bladder outlet obstruction index in mLUTS patients.14, 15 Unfortunately, in our study, detailed investigations of urodynamic parameters, including the evaluation of the presence of bladder outlet obstruction and detrusor overactivity, have not been performed. Therefore, our study design cannot clarify the reasons for the apparent differences in uroflowmetric results. However, we speculate that patient background and treatment protocol, including variables such as age, prostate volume, as well as dosage and duration of tadalafil administration, may affect the objective efficacy of tadalafil in these patients. As described above, we did not find the mechanism of Qmax improvement after tadalafil treatment. However, randomized, double‐blind placebo control studies15, 16 did not report similar results, and our results should be carefully considered.
One of the most interesting results of this study is that urinary 8‐OHdG concentration, normalized by urinary CR level is inversely associated with the efficacy of tadalafil in mLUTS patients with OAB. With regard to the pathological significance of 8‐OHdG, there is a general agreement that it is one of the useful markers of oxidative stress under various pathological conditions.17, 18 It should be noted that urinary levels of 8‐OHdG were increased in a rat model of atherosclerosis‐induced chronic bladder ischemia, which relates to oxidative stress.19 In addition, it has been reported that the severity of total OABSS, nocturia, and urge incontinence positively correlated with the urinary level of 8‐OHdG.8 These observations justify the use of the urinary 8‐OhdG level for the evaluation of oxidataive stress in our study. Although the objective findings such as VV and Qmax positively related to the urinary 8‐OHdG level, we could not find any relationship between the severity of OAB symptoms and urinary 8‐OHdG level prior to the administration of tadalafil in this study. The reason why the severity of subjective symptoms does not correlate with 8‐OHdG values remains unclear. However, compared to the study of Matsumoto et al,8 in which 8‐OHdG levels positively correlated with OAB symptoms, this study included more patients with relatively mild OAB symptoms and older patients than the prior study. In addition, in this study, compared with the data from the prior study,8 comparatively many patients with lifestyle‐related diseases, such as hypertension, diabetes, and renal dysfunction that would affect urinary 8‐OHdG levels were included. These might be the reasons why the urinary 8‐OHdG level did not correlate with the severity of OAB symptoms.
Tadalafil is commonly used for the treatment of mLUTS patients. Tadalafil improves LUTS by relaxing smooth muscle via its effect on the NO/cGMP/PDE5 pathway and by improving the regulation of bladder fusion, nerve activity, and smooth muscle proliferation.1, 2, 3, 4, 5 In addtion to such mechanisms, tadalafil also suppresses oxidative stress under a variety of pathological conditions.9, 10, 11 Furthermore, several reports showed that bladder ischemia, increased bladder nerve activity, intravesical pressure, and bladder aging that are prominent in several kinds of LUTS, including OAB, were accompanied by oxidative stress.19, 20, 21, 22 In addition, it has been previously shown that tadalafil decreased immunohistological staining for 8‐OHdG in the urothelial layer in a diabetic rat model.23 These findings suggest that tadalafil may reduce the level of oxidative stress in patients with urinary symptoms, including LUTS. However, the relationship between tadalafil treatment and urinary 8‐OHdG level in these patients has not been investigated previously. Thus, the present study for the first time reports that oxidative stress, as evaluated by urinary 8‐OHdG levels, is signficantly decreased by tadalafil treatment, and its change is associated with the efficacy of tadalafil treatment in mLUTS patients with OAB.
The major limitations of the present study are the open label rather than the placebo‐controlled design and a relatively small number of the patients. In addition, the observation period was relatively short. Therefore, a more detailed investigation, including multivariate analysis with a larger study population, is necessary to confirm our results. Furthermore, it should be noted that besides 8‐OHdG, other urinary markers, such as 8‐iso‐prostaglandin F2α or 4‐hydroxy‐2‐nonenal‐mercapturic acid, may be useful for the evaluation of oxidative stress.24 Further studies that will utilize other urinary markers are necessary to confirm our results. In addition, more detailed investigations of the molecular mechanisms of pharmacological effects of tadalafil are also important. For example, it has been reported that antifibrotic effects of green tea catechins improved the objective urinary symptoms in an OAB rat model,25 and tadalafil has been shown to have antifibrotic effects on urinary bladder remodeling in rats with spinal cord injury.26 Thus, our results hint at just some of the complex mechanisms of tadalafil pharmacological effects. On the other hand, we also think that the data on the outcome and changes in the urinary 8‐OHdG level following a 4‐week treatment with tadalafil are important based on previous reports.16, 27 However, despite these limitations, the relationship between the changes in urinary symptoms and urinary levels of an oxidative stress biomarker as a result of tadalafil treatment has been evaluated in the present study for the first time in mLUTS patients with OAB. Our findings are important for the understanding of mLUTS pathological characteristics at the molecular level and for the planning of treatment of these patients.
In conclusion, tadalafil administration improved urinary symptoms and increased VV and Qmax in mLUTS patients. Furthermore, urinary 8‐OHdG concentration normalized by the CR level was inversely proportional to the efficacy of the treatment. Our data suggest that tadalafil improved urinary symptoms by the downregulation of oxidative stress in mLUTS patients with OAB.
DISCLOSURE
None.
CONFLICTS OF INTEREST
None.
Matsuo T, Miyata Y, Araki K, et al. Efficacy of Tadalafil Therapy and Changes in Oxidative Stress Levels in Male Patients with Lower Urinary Tract Symptoms and Overactive Bladder. Lower Urinary Tract Symptoms. 2020;12:47–53. 10.1111/luts.12283
REFERENCES
- 1. Wang C. Phosphodiesterase‐5 inhibitors and benign prostatic hyperplasia. Curr Opin Urol. 2010;20:49‐54. [DOI] [PubMed] [Google Scholar]
- 2. Kedia GT, Uckert S, Jonas U, Kuczyk MA, Burchardt M. The nitric oxide pathway in the human prostate: clinical implications in men with lower urinary tract symptoms. World J Urol. 2008;26:603‐609. [DOI] [PubMed] [Google Scholar]
- 3. Morelli A, Sarchielli E, Comeglio P, et al. Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J Sex Med. 2011;8:2746‐2760. [DOI] [PubMed] [Google Scholar]
- 4. Minagawa T, Aizawa N, Igawa Y, Wyndaele JJ. Inhibitory effects of phosphodiesterase 5 inhibitor, tadalafil, on mechanosensitive bladder afferent nerve activities of the rat, and on acrolein‐induced hyperactivity of these nerves. BJU Int. 2012;110:E259‐E266. [DOI] [PubMed] [Google Scholar]
- 5. Adolfsson PI, Ahlstrand C, Varenhorst E, Svensson SPS. Lysophosphatidic acid stimulates proliferation of cultured smooth muscle cells from human BPH tissue: sildenafil and papaverin generate inhibition. Prostate. 2002;51:50‐58. [DOI] [PubMed] [Google Scholar]
- 6. Nomiya M, Yamaguchi O, Andersson KE, et al. The effect of atherosclerosis‐induced chronic bladder ischemia on bladder function in the rat. NeurourolUrodyn. 2012;31:195‐200. [DOI] [PubMed] [Google Scholar]
- 7. Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB. Overactivity and structural changes in the chronically ischemic bladder. J Urol. 1999;162:1768‐1778. [PubMed] [Google Scholar]
- 8. Matsumoto T, Hatakeyama S, Imai A, et al. Relationship between oxidative stress and lower urinary tract symptoms: results from a community health survey in Japan. BJU Int. 2019;123:877‐884. [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Li XX, Lin HC, et al. The effects of long‐term administration of tadalafil on STZ‐induced diabetic rats with erectile dysfunction via a local antioxidative mechanism. Asian J Androl. 2012;14:616‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koka S, Das A, Salloum FN, Kukreja RC. Phosphodiesterase‐5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic Biol Med. 2013;60:80‐88. [DOI] [PubMed] [Google Scholar]
- 11. Ameli M, Hashemi MS, Moghimian M, Shokoohi M. Protective effect of tadalafil and verapamil on testicular function and oxidative stress after torsion/detorsion in adult male rat. Andrologia. 2018;19:e13068. [DOI] [PubMed] [Google Scholar]
- 12. Matsumoto S, Hanai T, Matsui T, Oka M, Tanaka M, Uemura H. Eviprostat suppresses urinary oxidative stress in a rabbit model of partial bladder outlet obstruction and in patients with benign prostatic hyperplasia. Phytother Res. 2010;24:301‐303. [DOI] [PubMed] [Google Scholar]
- 13. Matsukawa Y, Majima T, Matsuo K, et al. Effects of tadalafil on storage and voiding function in patients with male lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a urodynamic‐based study. Int J Urol. 2018;25:246‐250. [DOI] [PubMed] [Google Scholar]
- 14. Roehrborn CG, Kaminetsky JC, Auerbach SM, Montelongo RM, Elion‐Mboussa A, Viktrup L. Changes in peak urinary flow and voiding efficiency in men with signs and symptoms of benign prostatic hyperplasia during once daily tadalafil treatment. BJU Int. 2010;105:502‐507. [DOI] [PubMed] [Google Scholar]
- 15. Dmochowski R, Roehrborn C, Klise S, Xu L, Kaminetsky J, Kraus S. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12‐week clinical trial. J Urol. 2013;189(1 Suppl):S135‐S140. [DOI] [PubMed] [Google Scholar]
- 16. Porst H, Kim ED, Casabé AR, et al. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double‐blind, placebo‐controlled trial. Eur Urol. 2011;60:1105‐1113. [DOI] [PubMed] [Google Scholar]
- 17. Ma‐On C, Sanpavat A, Whongsiri P, et al. Oxidative stress indicated by elevated expression of Nrf2 and 8‐OHdG promotes hepatocellular carcinoma progression. Med Oncol. 2017;34:57. [DOI] [PubMed] [Google Scholar]
- 18. Paredes‐Sánchez E, Montiel‐Company JM, Iranzo‐Cortés JE, Almerich‐Torres T, Bellot‐Arcís C, Almerich‐Silla JM. Meta‐analysis of the use of 8‐OHdG in saliva as a marker of periodontal disease. Dis Markers. 2018;2018:7916578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsui T, Oka M, Fukui T, et al. Suppression of bladder overactivity and oxidative stress by the phytotherapeutic agent, Eviprostat, in a rat model of atherosclerosis‐induced chronic bladder ischemia. Int J Urol. 2012;19:669‐675. [DOI] [PubMed] [Google Scholar]
- 20. Yang JH, Siroky MB, Yalla SV, Azadzoi KM. Mitochondrial stress and activation of PI3K and Akt survival pathway in bladder ischemia. Res Rep Urol. 2017;9:93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nocchi L, Daly DM, Chapple C, Grundy D. Induction of oxidative stress causes functional alterations in mouse urothelium via a TRPM8‐mediated mechanism: implications for aging. Aging Cell. 2014;13:540‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sezginer EK, Yilmaz‐Oral D, Lokman U, et al. Effects of varying degrees of partial bladder outlet obstruction on urinary bladder function of rats: a novel link to inflammation, oxidative stress and hypoxia. Low Urin Tract Symptoms. 2019;11:193‐201. [DOI] [PubMed] [Google Scholar]
- 23. Gotoh D, Torimoto K, Tatsumi Y, et al. Tadalafil, a phosphodiesterase type 5 inhibitor, improves bladder blood supply and restores the initial phase of lower urinary tract dysfunction in diabetic rats. NeurourolUrodyn. 2018;37:666‐672. [DOI] [PubMed] [Google Scholar]
- 24. Wang YX, Liu C, Shen Y, et al. Urinary levels of bisphenol a, F and S and markers of oxidative stress among healthy adult men: variability and association analysis. Environ Int. 2018;123:301‐309. [DOI] [PubMed] [Google Scholar]
- 25. Juan YS, Chuang SM, Lee YL, et al. Green tea catechins decrease oxidative stress in surgical menopause‐induced overactive bladder in a rat model. BJU Int. 2012;110:E236‐E244. [DOI] [PubMed] [Google Scholar]
- 26. Kadekawa K, Majima T, Kawamorita N, et al. Effects of an alpha1A/D‐adrenoceptor antagonist, naftopidil, and a phosphodiesterase type 5 inhibitor, tadalafil, on urinary bladder remodeling in rats with spinal cord injury. NeurourolUrodyn. 2017;36:1488‐1495. [DOI] [PubMed] [Google Scholar]
- 27. Brock G, Broderick G, Roehrborn CG, Xu L, Wong D, Viktrup L. Tadalafil once daily in the treatment of lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) in men without erectile dysfunction. BJU Int. 2013;112:990‐997. [DOI] [PubMed] [Google Scholar]


