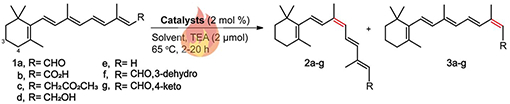
Table 1.
Transition metal-catalyzed Z-isomerization of all-trans-retinoid using traditional heat treatment.a,b
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Sub | Solvent | Time | Conversionc (%) | Selectivity of 9-cisd (%) | Product (3:2) | Ratio range | Effective Cat |
| 1 | 1a | C6H14 | 20 h | 33-39 | 37-44 | 13-cis: 9-cis | 1.2- 1.6: 1 | I-V, VII |
| 2 | 1b | C6H14 | 20 h | 15-34 | 21-98 | 13-cis: 9-cis | 0- 3.9: 1 | I, III-V, VII |
| 3 | 1c | C6H14 | 20 h | 18-24 | 85-90 | 13-cis: 9-cis | 0.1- 5.6: 1 | I-V, VII |
| 4 | 1d | C6H14 | 20 h | 16-21 | 77-94 | 13-cis: 9-cis | 0.1- 0.6: 1 | II, IV, VII |
| 5 | 1e | CH3CN | 2 h | 24 | 83 | 12-cis: 8-cis | 0.2: 1 | I |
| 6 | 1f | CH3CN | 2 h | 43-44 | 39-45 | 13-cis: 9-cis | 0- 3: 1 | I-V, XIX |
| 7 | 1g | CH3CN | 2 h | 33-46e | 45-55 | 13-cis: 9-cis | 0- 1.05: 1 | I-XI |
Reactions were carried out with all-trans-retinoid (0.1 mmol), catalyst (2 mol %), and TEA (0.2 μmol) at 65 °C under N2.
E/Z ratios reported are the average of two experiments and were determined by HPLC analysis.
Conversion to Z-isomer of the most effective catalysts.
Yields of 9-cis isomer for the most effective catalysts. 4.4 % of 9,13-dicis was detected. No reaction occurred without a catalyst for all substrates.
