Abstract
Metastasis is a critical determinant for the treatment strategy and prognosis in patients with squamous cell carcinoma of the head and neck (SCCHN). However, the mechanisms underlying SCCHN metastasis are poorly understood. Our study sought to determine the key microRNA and their functional mechanisms involved in SCCHN metastasis. For The Cancer Genome Atlas (TCGA) data analysis, quantitative PCR was used to quantify the level of miR‐30e‐5p in SCCHN and its clinical significance was further analyzed. A series of in vitro and in vivo experiments were applied to determine the effects of miR‐30e‐5p and its target AEG‐1 on SCCHN metastasis. A mechanism investigation further revealed that AEG‐1 was implicated in the angiogenesis and metastasis mediated by miR‐30e‐5p. Overall, our study confirms that miR‐30e‐5p is a valuable predictive biomarker and potential therapeutic target in SCCHN metastasis.
Keywords: AEG‐1, angiogenesis, metastasis, miR‐30e‐5p, squamous cell carcinoma of the head and neck
Valuable predictive biomarkerPotential therapeutic targetMetastasis related gene
1. INTRODUCTION
Squamous cell carcinoma of head and neck (SCCHN) arises, for example, in the oral cavity, the nasopharynx, the oropharynx, the hypopharynx and the larynx. It represents the sixth leading cancer by incidence and there are approximately 500 000 new patients around the world annually.1 Patients with SCCHN suffer from vital dysfunctions, including dyspnea, dysphagia and dysphonia. Although multiple therapies including surgery, radiotherapy and chemotherapy have been applied to fight against SCCHN for decades, the prognosis for patients with SCCHN remains unsatisfactory.2 Local and/or distant metastasis definitely contributes to the poor prognosis in patients with SCCHN.3, 4 Therefore, it is urgent and imperative to clarify the mechanisms underlying metastasis, which will benefit future surveillance and target therapy to overcome SCCHN metastasis.
Genetic aberrations in cancer cells are reprogrammed to acquire aggressive traits such as epithelial‐mesenchymal transition (EMT).5 EMT is a process in which cancer cells lose their polarity and cell‐cell adhesion, change to mesenchymal fibroblast‐like cells, and, finally, confer metastatic properties upon cancer cells through enhancing migratory and invasive abilities.5, 6 In contrast, endogenous oncogenes or suppressors also shape the tumor microenvironment (TME) to facilitate cancer metastasis.7, 8 As a critical component in the TME, new formation of blood vessels in tumors, called angiogenesis, is essential to provide nutrition and oxygen to support the malignant cancer growth and also generate the metastatic vessel for cancer cells to spread.9 Cancer cells secrete distinct angiogenic growth factors and activate their corresponding receptors in TME to promote cancer angiogenesis.10 Thus, angiogenesis is important in cancer metastasis,11 indicating the potential of antiangiogenic therapy.12 However, the molecular regulatory mechanisms involved in angiogenesis and cancer metastasis are complicated, which should be addressed in future.
MicroRNA (miRNA) are a family of small non‐coding RNA that are involved in the post–transcriptional modulation of target genes. MiRNA bind to the 3′‐ or 5′‐untranslated regions (UTR) of target mRNA, leading to the degradation of mRNA and repression of protein translation.13, 14, 15 It is well established that miRNA play key roles in diverse biopathological behaviors, including malignant growth, metastasis and resistance to chemotherapy and radiotherapy.13, 14 Abundant studies have shown that miRNA are dysregulated in numerous cancers, including SCCHN, contributing to cancer progression.16, 17 Therefore, modulation of specific miRNA activities represents a potential therapeutic avenue.13, 15
Here, the Cancer Genome Atlas (TCGA) data and a validation patient cohort were used to determine that miR‐30e‐5p was closely associated with the aggressive traits of SCCHN, including metastasis. Survival analysis revealed that patients with low expression of miR‐30e‐5p displayed a worse prognosis, and miR‐30e‐5p was an independent factor for the survival prognosis in patients with SCCHN. Further in vitro and in vivo evidence demonstrated that miR‐30e‐5p, as a metastasis suppressor, obviously repressed the migration, invasion and metastasis of SCCHN. Mechanistically, miR‐30e‐5p inhibited the occurrence of EMT of SCCHN cells, and also blocked SCCHN angiogenesis through downregulating multiple proangiogenic factors, including vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF). More importantly, we found that oncogene AEG‐1 was directly targeted by miR‐30e‐5p and partially involved in the angiogenesis and metastasis mediated by miR‐30e‐5p. Our data suggest that miR‐30e‐5p is a valuable prognosis biomarker and a potential therapeutic target for SCCHN metastasis.
2. MATERIALS AND METHODS
2.1. Cell culture
Squamous cell carcinoma of the head and neck JHU011, CAL33 and CAL27 cell lines were kindly provided by Dr Joseph Califano (University of California, San Diego, USA). Tu686 cell line was kindly provided by Dr Zhuo G. Chen (Emory University Winship Cancer Institute, USA).18 Tca8113 cell line was obtained from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences. The FaDu and the HUVEC were purchased from ATCC. SCCHN cells were cultured in appropriate media supplemented with essential materials.
2.2. The Cancer Genome Atlas data analysis
Differentially expressed miRNA profile based on TCGA data was obtained from OncomiR (http://www.oncomir.org), in which 522 SCCHN and 44 noncancerous samples were included.
2.3. Human squamous cell carcinoma of head and neck tissue samples
All SCCHN tissue samples and clinical information of patients were collected in the same manner as in our previous studies.19, 20 The study was approved by the Research Ethics Committee of Central South University, Changsha, China.
2.4. Quantitative RT‐PCR analysis
cDNA was reversed from 1 μg total qualified RNA using the All‐in‐One miRNA or mRNA cDNA Synthesis Kit (GeneCopoeia) following the manufacturer’s protocol. Relative gene expression values were calculated using the 2−△△CT method and normalized with a control (U6 for miRNA and GAPDH for mRNA).21 The primers are listed in Table S1.
2.5. Transfection
Squamous cell carcinoma of head and neck cells were transfected with the miR‐30e‐5p mimic/negative control (NC) or inhibitor/inhibitor NC (GenePharma) according to the manufacturer’s protocol. In addition, the empty vector and miR‐30e‐5p overexpression plasmid (GeneCopoeia) were used for stable expression of miR‐30e‐5p in Fadu cells. For AEG‐1 downexpression, a lentiviral vector encoding AEG‐1sh was used (GeneCopoeia) at a multiplicity of infection (MOI) of 50 pfu/cell.
2.6. Wounding‐healing and transwell invasion assay
Wound‐healing and transwell invasion assays were performed as we described previously.19, 20
2.7. Colony formation assay
Squamous cell carcinoma of head and neck cell colonies were stained 2 weeks later and counted by Image J v1.8.0 (positive colony was defined as >50 cells).22
2.8. Western blotting assay
The western blot assay was carried out according to our previous studies.19, 20, 22, 23 Antibody information is listed in Table S2.
2.9. Immunofluorescence microscopy analysis
The cells in slides were incubated with a rabbit anti–human primary antibody against E‐cadherin (Santa Cruz) or mouse anti–human antibody against vimentin (Proteintech Group) at 4°C overnight. Next, the cells were incubated in the dark box with Alexa Fluo 488 goat anti–rabbit IgG (H+L) and Alexa Fluor 594 goat anti–mouse IgG (Jackson ImmunoResearch).
2.10. Endothelial cell tube formation assay
The 100 μL serum‐free supernatant mixed with 1 × 104 HUVEC cells were seeded in 96‐well plates that spread with 50 μL of high concentration Matrigel (BD Biosciences). Five random selected fields of view were captured using a microscope at 4 hours after implantation.
2.11. Chick chorioallantoic membrane assay
Thirteen fertilized chicken eggs were incubated for 8 days. Then a small circular window was opened above the air sac, which was followed by implantation of 20 μL SCCHN cells (2 × 106 cells) mixed with 20 uL of high concentration Matrigel (BD Biosciences) into chick chorioallantoic membrane (CAM) for each egg. Images of CAM were observed 72 hours after implantation.
2.12. Matrigel plug angiogenesis assay
Fadu cells suspended in 100 μL PBS (100 μL) were mixed with 100 μL of high concentration Matrigel. Then the mixed liquor was injected subcutaneously into hind flanks of nude mice. One week after inoculation, the gel plugs were collected.
2.13. Xenograft tumor model
Fadu cell aliquots (200 μL) of 4.0 × 106 cells were injected subcutaneously into 4‐week‐old male BALB/c nude mice (five mice per group) in their right flank. Approximately 4 weeks later, the mice were killed by anesthesia and tumor samples were harvested.
2.14. Metastasis model
To establish the metastasis model, 4 × 106 Fadu cells suspended in 200 μL PBS were injected via the tail vein in 4‐week‐old male BALB/c nude mice (five mice per group). After 6 weeks, liver and lung tissues were fixed and stained with H&E.
2.15. H&E staining, immunohistochemistry and quantification
All H&E staining and immunohistochemistry staining was conducted as previously described.24, 25, 26
2.16. Luciferase reporter assays
Fadu and JHU011 cells were seeded on 24‐well plates and cotransfected with 50 nmol/L of either miR‐30e‐5p mimics or NC oligos together with 200 ng plasmid of wild‐type or mutated 3′‐UTR of the AEG‐1 (GeneCopoeia) using FuGENE 6 Reagent (Promega). Two days later, relative luciferase activity was measured using the Dual‐Luciferase Reporter Assay System (Promega).
2.17. Statistical analysis
All data were analyzed using SPSS 20.0. Results in this study were presented as the mean ± SD. The statistical comparisons of two groups were determined by Student’s t test (for equal variances) or Mann–Whitney U test (for unequal variances). In addition, survival curves were plotted using the Kaplan–Meier method and compared using the log‐rank test. P < 0.05 was regarded as statistically significant.
3. RESULTS
3.1. Aberrant expression profile of miRNA in patients with squamous cell carcinoma of the head and neck
To comprehensively analyze the aberrant expression of miRNA in patients with SCCHN, TCGA sequencing data including 522 SCCHN and 44 noncancerous samples were used to delineate a whole aberrant expression profile of miRNA (Table S3). As a result, 376 miRNA were dysregulated in SCCHN samples and 172 miRNA were found to be predictive in patients’ survival analysis (Figure S1A; Table S3). Among these miRNA, 33 abnormally expressed miRNA, including 16 upregulated and 17 downregulated miRNA, were determined to have prognosticative value (Figure S1A). Finally, 2 miRNA (miR‐30e‐5p and miR‐3187‐3p) were ultimately analyzed and found to be associated with SCCHN metastasis (Figure S1B). These data demonstrate that these 2 miRNA may be potential biomarkers for prognosis and molecular targets for SCCHN metastasis. However, in our current study, we only focus on the significance and biological effects of miR‐30e‐5p in SCCHN.
3.2. Low expression of miR‐30e‐5p predicts a worse prognosis in patients with squamous cell carcinoma of the head and neck
To further validate the clinical significance of miR‐30e‐5p in patients with SCCHN, the relative expression of miR‐30e‐5p was quantified in 113 cases of SCCHN via quantitative PCR (qPCR). Our data revealed that miR‐30e‐5p was dramatically decreased in SCCHN samples compared with corresponding adjacent noncancerous tissues (Figure 1A; P < 0.01). For further analysis, our SCCHN patient cohort was divided into two groups: high miR‐30e‐5p (n = 56) and low miR‐30e‐5p (n = 57) expression groups, in which the expression level of miR‐30e‐5p was greater than or less than the median value in 113 patients with SCCHN. As summarized in Table 1 via Student’s t test, low expression of miR‐30e‐5p was closely associated with high T classification, advanced clinical stage and cervical lymph node metastasis in patients with SCCHN (Table 1; all P < 0.05). Importantly, survival analysis further indicated that patients with low expression of miR‐30e‐5p displayed a worse prognosis than that in patients with high expression of miR‐30e‐5p (Figure 1B; P < 0.001). Univariate Cox regression analyses revealed that histological grade, metastasis and status of miR‐30e‐5p expression were significantly associated with the overall survival status (Table 2; all P < 0.05). Furthermore, histological stage, metastasis and miR‐30e‐5p expression were determined to be independent factors with prognostic value for the overall survival in patients with SCCHN in the multivariate Cox regression analyses (Table 2). Taken together, these data suggest that miR‐30e‐5p is a valuable biomarker for prognosis in patients with SCCHN.
Figure 1.
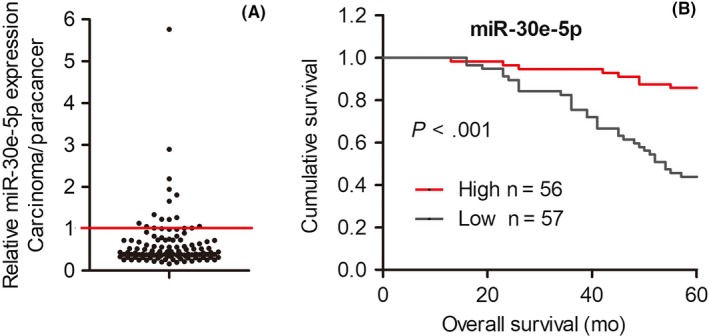
Correlations between expression of miR‐30e‐5p and prognosis. A, quantitative RT‐PCR results showed that the relative expression of miR‐30e‐5p was downregulated in squamous cell carcinoma of head and neck (SCCHN) tissue samples compared with corresponding adjacent noncancerous tissues. The unit in the Y‐axis indicates the ratio of carcinoma/paracancer. B, Kaplan‐Meier analysis in patients with SCCHN according to expression level of miR‐30e‐5p. ***P < 0.001
Table 1.
Correlations between miR‐30e‐5p expression and clinicopathological parameters in squamous cell carcinoma of head and neck (SCCHN) patients
| Parameters | Number of patients | miR‐30e‐5p expression | t value | P‐value* |
|---|---|---|---|---|
| Age | ||||
| <59 | 60 | 0.512 ± 0.284 | −2.277 | 0.025 |
| ≥59 | 53 | 0.785 ± 0.881 | ||
| Gender | ||||
| Female | 8 | 0.715 ± 0.535 | 0.338 | 0.736 |
| Male | 105 | 0.634 ± 0.659 | ||
| Smoking | ||||
| Yes | 69 | 0.527 ± 0.311 | 2.352 | 0.020 |
| No | 44 | 0.817 ± 0.946 | ||
| Histological grade | ||||
| G1 + G2 | 45 | 0.602 ± 0.452 | −0.501 | 0.617 |
| G3 | 68 | 0.665 ± 0.755 | ||
| T classification | ||||
| T1 + T2 | 60 | 0.756 ± 0.808 | 20.047 | 0.043 |
| T3 + T4 | 53 | 0.509 ± 0.367 | ||
| Clinical stage | ||||
| I + II | 42 | 0.871 ± 0.930 | 3.007 | 0.003 |
| III + IV | 71 | 0.503 ± 0.342 | ||
| Lymph node metastasis | ||||
| N0 | 66 | 0.807 ± 0.796 | 3.390 | 0.001 |
| N+ | 47 | 0.405 ± 0.187 | ||
P < 0.05 was considered to be statistically significant (in bold and italics).
Table 2.
Cox model analysis of overall survival
| Parameters | Relative risk (95% CI) | P‐value |
|---|---|---|
| Univariate | ||
| Age | 1.895 (0.969‐3.707) | 0.062 |
| Gender | 0.983 (0.329‐2.941) | 0.976 |
| Smoking | 0.661 (0.307‐1.426) | 0.291 |
| T classification | 1.027 (0.984‐1.073) | 0.224 |
| Clinical stage | 1.026 (0.956‐1.101) | 0.480 |
| Histological grade | 2.111 (1.034‐4.311) | 0.040 |
| Lymph node metastasis | 3.069 (1.185‐7.947) | 0.021 |
| miR‐30e‐5p expression | 0.236 (0.096‐0.577) | 0.002 |
| Multivariate | ||
| Lymph node metastasis | 4.170 (1.996‐8.713) | 0.000 |
| miR‐30e‐5p expression | 0.222 (0.099‐0.498) | 0.000 |
| Histological grade | 2.168 (1.110‐4.233) | 0.023 |
All the clinicopathological variables listed in the table were included in the univariate and multivariate analyses.
95% CI, 95% confidence interval.
P < 0.05 was considered to be statistically significant (in bold and italics).
3.3. miR‐30e‐5p impedes squamous cell carcinoma of the head and neck metastasis
To further investigate the function of miR‐30e‐5p in the progression of SCCHN, its expression was modulated in diverse SCCHN cell lines, and then alterations in cell malignant behaviors were examined. Initially, expression of miR‐30e‐5p was quantified in 6 SCCHN cell lines, in which its expression was high in Tu686, and low in Fadu and JHU011 (Figure S2A). Therefore, its expression was 20‐30‐fold overexpressed in Fadu and JHU011 cell lines, and >80% inhibited in Tu686 cells (Figure S2B). Plate cloning formation assays were then used to check the proliferative changes, which indicated that miR‐30e‐5p did not influence the proliferation of SCCHN cell lines in vitro (Figure S2C,D). We also stably overexpressed miR‐30e‐5p in Fadu cells and established the xenograft tumors in nude mice (Figure S2E). The in vivo experiments further confirmed that miR‐30e‐5p had no effects on the growth pattern of SCCHN cells (Figure S2F‐H).
Based on the results that miR‐30e‐5p was closely associated with SCCHN metastasis in clinical patient samples, both wound‐healing and Transwell assays were used to check the effects of miR‐30e‐5p on migration and invasion in vitro. As presented in Figure 2A‐2D, ectopic overexpression of miR‐30e‐5p dramatically repressed the migratory and invasive abilities of both Fadu and JHU011 cells in vitro. In contrast, miR‐30e‐5p inhibition led to enhanced migration and invasion of Tu686 cells in vitro (Figure 2A‐D). For the reason that these SCCHN cell lines cannot form metastatic lesions after subcutaneous tumorigenesis, Fadu cells were injected via mouse tail veins to form distant organ metastatic lesions (Figure 2E). Our data based on this metastatic model indicated that miR‐30e‐5p impeded the formation of lung metastatic lesions of Fadu cells (Figure 2F,G). Both the size and number of lung metastatic tumors were reduced in Fadu cells with miR‐30e‐5p overexpression (Figure 2H,I). Notably, no liver metastatic lesions were observed in mice after the delivery of lenti‐miR‐30e‐5p or lenti‐miR‐ctrl (Figure S2I). Collectively, these data indicate that miR‐30e‐5p, as a metastasis suppressor, inhibits the metastasis of SCCHN cells but not malignant proliferation, suggesting that miR‐30e‐5p is a metastasis‐associated gene.
Figure 2.
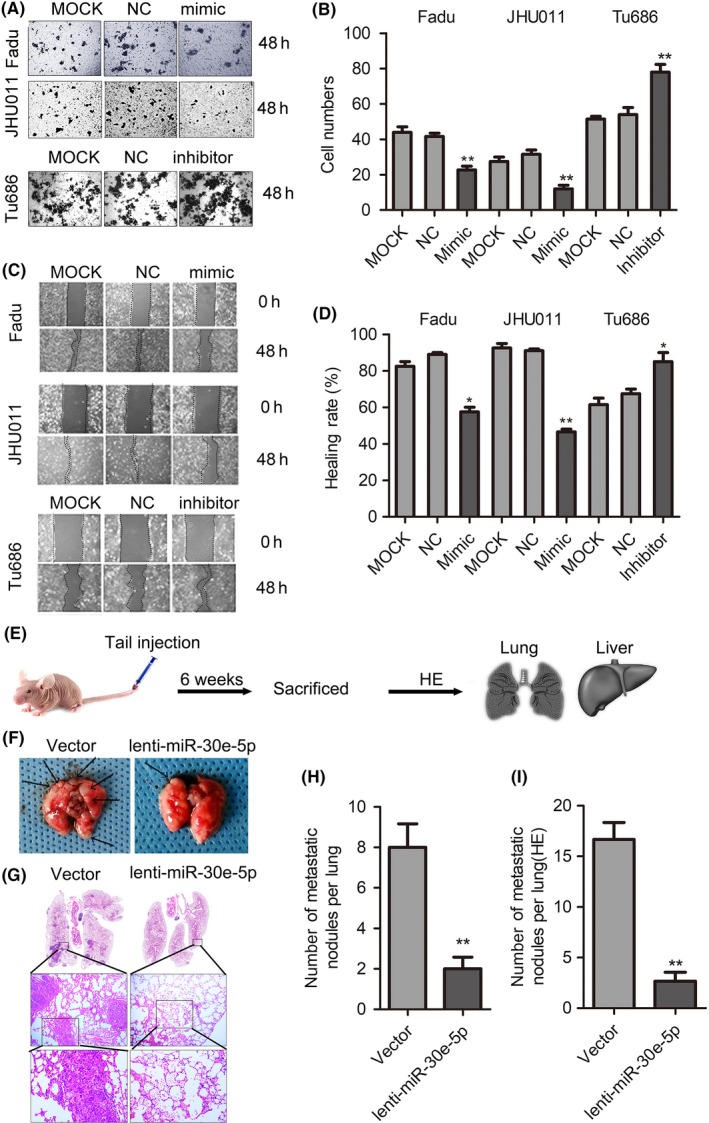
miR‐30e‐5p impedes squamous cell carcinoma of head and neck (SCCHN) metastasis. A and B, Cell invasion was assessed using a transwell assay. Representative fields with migrated cells (A) and quantification of the number of migration cells (B) are shown in the insets. C and D, The scratch test was used to check the changes in healing ability in vitro (C) and the percentage of healing area was determined (D). “The healing rate” is the ratio of “healing area” to “area of original wound.” E, Distant lung metastatic model was established via the tail vein injection of Fadu cells that stably overexpressed miR‐30e‐5p or vector. F and H, The images of macroscopic lung metastatic nodules were photographed (F), and numbers of lung nodules were calculated (H); arrowheads indicate metastatic nodules. G and I, Representative images (G) and quantification (I) of microscopic lung metastatic nodules stained with H&E. *P < 0.05; **P < 0.01
3.4. miR‐30e‐5p inhibits epithelial‐mesenchymal transition and angiogenesis in squamous cell carcinoma of head and neck
Cancer malignant progression is determined by both endogenous changes of oncogenes and/or suppressors, and its surrounding tumor microenvironment (TME).7, 8 In the aspect of cancer cells, EMT is a complicated reprogramming process, which is characterized by the inhibition of epithelial markers and upregulation of mesenchymal markers.6, 16 Therefore, we used multiple methods to evaluate the effects of miR‐30e‐5p on the alterations of EMT markers. As shown in Figure 3A, the qPCR results revealed that miR‐30e‐5p overexpression in Fadu and JHU011 obviously increased the mRNA expression of E‐cadherin and reduced the mRNA expression of vimentin, which was accompanied by the inhibition of EMT transcription factor including mRNA of Twist 1 and Twist 2 (Figure 3A). Western blotting and immunofluorescence staining confirmed that miR‐30e‐5p overexpression in Fadu and JHU011 also upregulated E‐cadherin and reduced vimentin (Figure 3B,C). Conversely, miR‐30e‐5p inhibition correspondingly repressed the expression of E‐cadherin and increased the expression of vimentin at both mRNA and protein levels (Figure 3A‐C). These data clearly demonstrate that miR‐30e‐5p inhibits the occurrence of EMT.
Figure 3.
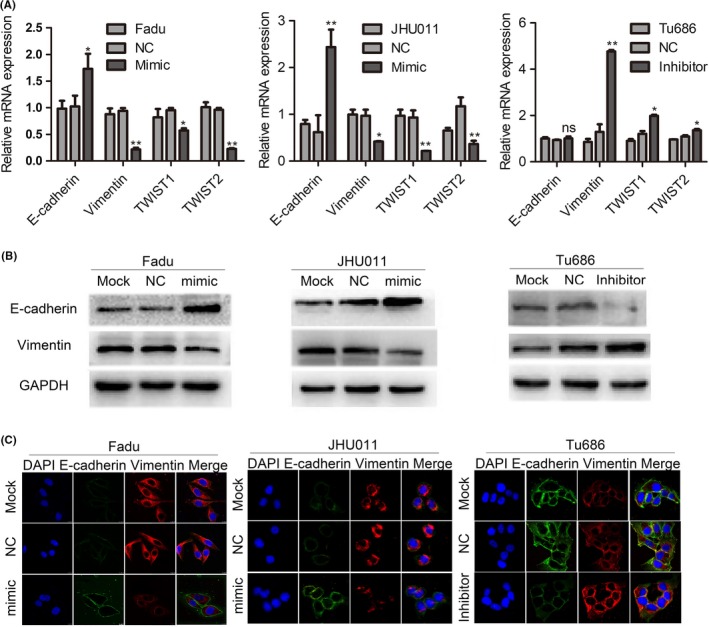
miR‐30e‐5p inhibits epithelial‐mesenchymal transition (EMT) in squamous cell carcinoma of head and neck (SCCHN). A, Quantitative PCR analysis quantified the expression of EMT markers of E‐cadherin, vimentin and related transcription factor, including Twist1 and Twist2 in Fadu, JHU011 and Tu686 cells. The 2−ΔΔCT method was used to measure the relative mRNA expression. B and C, Representative western blotting (B) and immunofluorescence staining (C) showed the expression of E‐cadherin and vimentin. *P < 0.05; **P < 0.01; ns, not significant
Angiogenesis has an important function in cancer metastasis, which provides a vessel for cancer cells to migrate into the blood system.9, 12, 27 In examining the exact role of miR‐30e‐5p in SCCHN angiogenesis, the migratory ability of HUVEC was found to be significantly suppressed when cocultured with Fadu cells that upregulated miR‐30e‐5p (Figure 4A,B). Consistent with migration assays, miR‐30e‐5p overexpression in Fadu and JHU011 cells inhibited capillary‐like tube formation of HUVEC cells in Matrigel (Figure 4C,D). To answer the question of how miR‐30e‐5p represses SCCHN angiogenesis, we quantified a panel of cytokines and chemokines involved in cancer angiogenesis, which revealed that miR‐30e‐5p overexpression significantly inhibited the mRNA expression of VEGF, HGF, FGF1, CSF1/2/3, FGF2, IL‐8, IGF1 and IGF2 (Figure 4E,F). This result clearly suggests that miR‐30e‐5p can exert a broad inhibitory effect on the expression of proangiogenic regulators.
Figure 4.
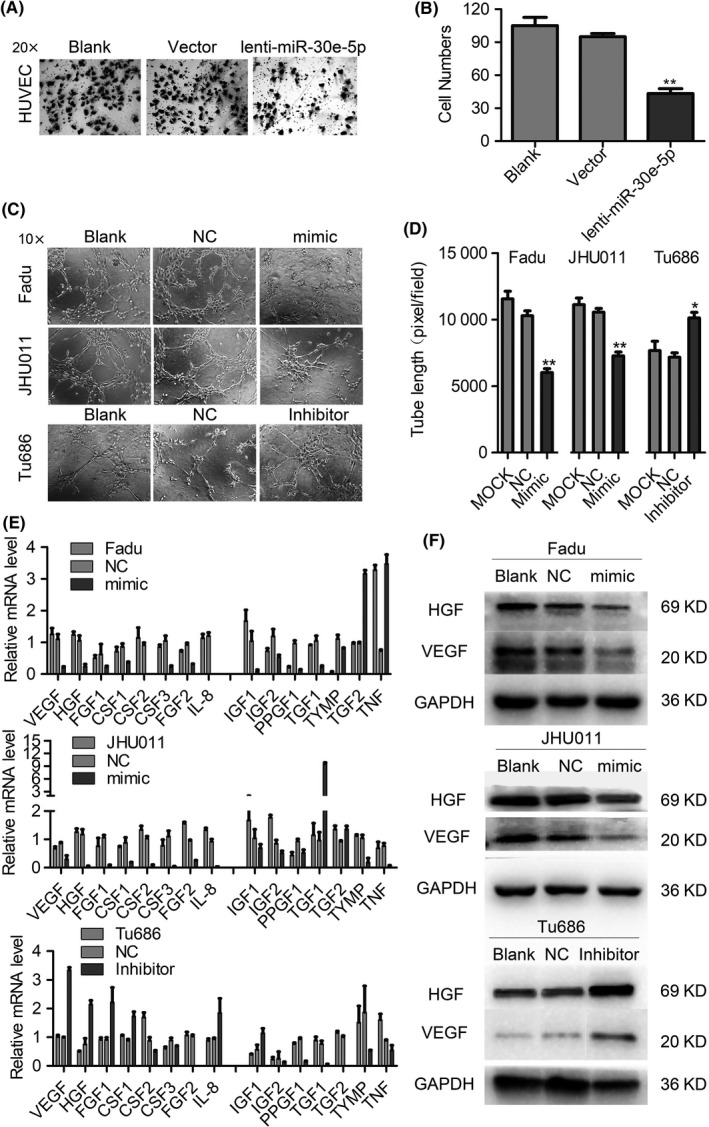
miR‐30e‐5p suppresses angiogenesis in squamous cell carcinoma of head and neck (SCCHN). A, The blood vessel epithelial cell HUVEC cocultured with Fadu cells transfected with miR‐30e‐5p mimic. B, Quantification of the number of migrated cells (B). C and D, Tube formation by HUVEC cells was measured and the results were expressed as the tubule length. Representative morphological images (C) and statistical results (D) are shown. E and F, The effects of miR‐30e‐5p on the expression levels of cytokines and chemokines involved in cancer angiogenesis measured by quantitative PCR (E) and western blot (F) analysis. The 2−ΔΔCT method was used to measure the relative mRNA expression. *P < 0.05; **P < 0.01
In examining the role of miR‐30e‐5p in cancer angiogenesis, in vivo Matrigel angiogenesis plug assay revealed that miR‐30e‐5p overexpression in Fadu cells reduced the tumors’ newly formed blood vessels in Matrigel (Figure 5A,B), which was accompanied by the decline of proangiogenic factors including VEGF, FGF1, CSF1/2/3 and HGF (Figure 5C). H&E staining in plug gels and xenograft tumors samples revealed that MVD in the group of miR‐30e‐5p overexpression was also reduced (Figure 5D‐J). In addition, immunostaining of proangiogenic factor VEGF and blood vessel epithelial marker CD31 were also significantly decreased in the miR‐30e‐5p overexpression group (Figure 5D‐J). Finally, the chick chorioallantoic membrane vascular assay indicated that miR‐30e‐5p overexpression in Fadu cells similarly reduced the vascular density (Figure 5K,L). Collectively, these data clearly indicate that miR‐30e‐5p represses EMT in cancer cells themselves and also impedes the formation of cancer angiogenesis.
Figure 5.
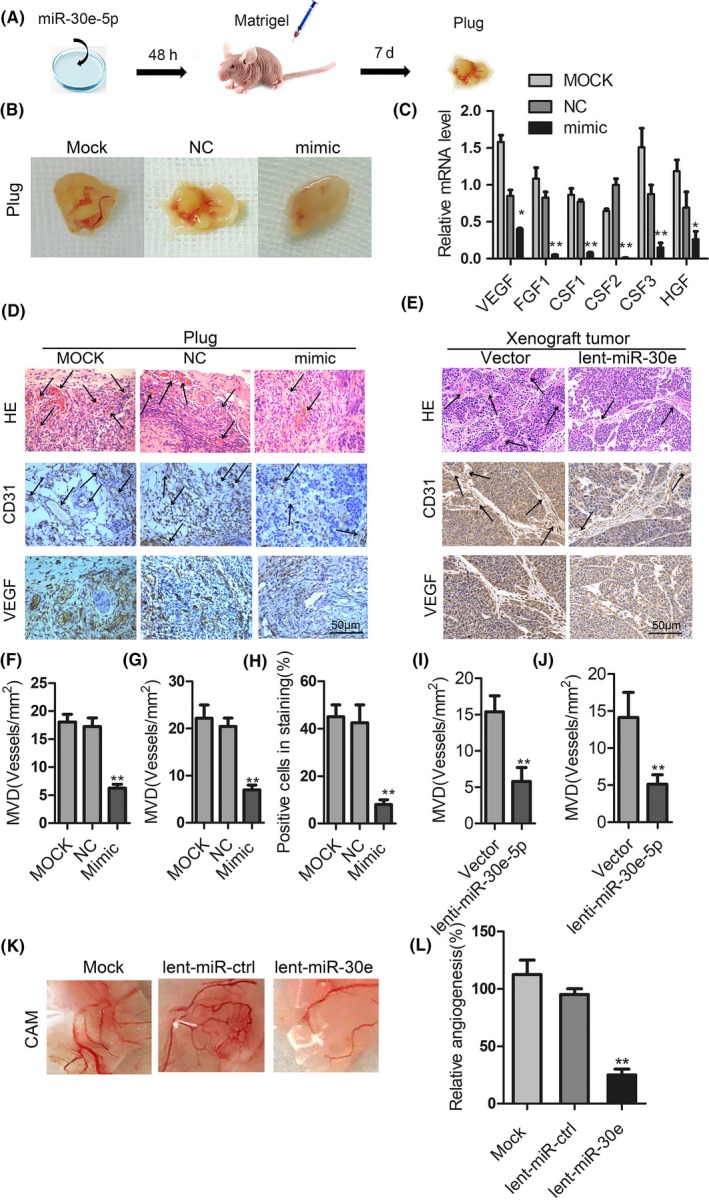
miR‐30e‐5p suppresses angiogenesis in squamous cell carcinoma of head and neck (SCCHN) in vivo. A, Matrigel angiogenesis plug assay was formed by subcutaneously implanting Fadu cells with Matrigel. B and C, Gel plugs were collected and photographed (B) in 7 d after implantation; the proangiogenic factors were detected by quantitative PCR detection (C). D‐J, H&E staining and immunohistochemical staining analysis of the levels of CD31 and vascular endothelial growth factor (VEGF) in gel plugs (D) and xenograft tumors (E) of nude mice. Arrows are pointed to neovascularization and quantification of the microvessel density (F, G, I, J). The positive staining cell numbers of CD31 were counted (H). K and L, chick chorioallantoic membrane (CAM) angiogenesis assays were performed with Fadu cells stably overexpressing miR‐30e‐5p or vector. Representative images of new blood vessel formation (K) and quantification of the average number of new blood vessels (L; n = 10 for each group). *P < 0.05; **P < 0.01
3.5. AEG‐1 mediates the effect of miR‐30e‐5p on angiogenesis and metastasis
To finally ascertain the potential target mRNA of miR‐30e‐5p, four online prediction algorithms were chosen to screen the potential targeted mRNA of miR‐30e‐5p: TargetScanHuman (targetscan.org), Pictar (pictar.org), miRNABD (sysbio.suda.edu.cn) and microT‐CDS (diana.imis.athena‐innovation.gr). As a result, five mRNA presented in the lists of four online prediction algorithms (Figure 6A). In addition, among these five mRNA, AEG‐1 ranked first on the list, and displayed an opposite expression style with miR‐30e‐5p based on the analysis of SCCHN TCGA data (Figure 6B; Figure S3). Therefore, we used luciferase reporter assays to determine whether AEG‐1 was a direct binding target of miR‐30e‐5p. Luciferase reporter plasmids encoding the wild‐type (WT) or mutant (MU) 3′‐UTR domain of AEG‐1 mRNA were designed (Figure 6C), and then miR‐30e‐5p mimic was cotransfected with the reporter plasmid into SCCHN Fadu and JHU011 cells. As shown in Figure6D, luciferase activities in Fadu and JHU011 cells cotransfected with AEG‐1 3′‐UTR‐WT and miR‐30e‐5p mimic were significantly lower than those cells cotransfected with AEG‐1 3′‐UTR‐WT and NC (Figure6D; P < 0.01). These data meant that miR‐30e‐5p reduced the expression of AEG‐1 by directly binding to its 3′‐UTR region of AEG‐1 mRNA. Consistent with the result of luciferase reporter assays, miR‐30e‐5p mimic also greatly inhibited the expression of AEG‐1 mRNA (Figure 6E) and protein (Figure 6F).
Figure 6.
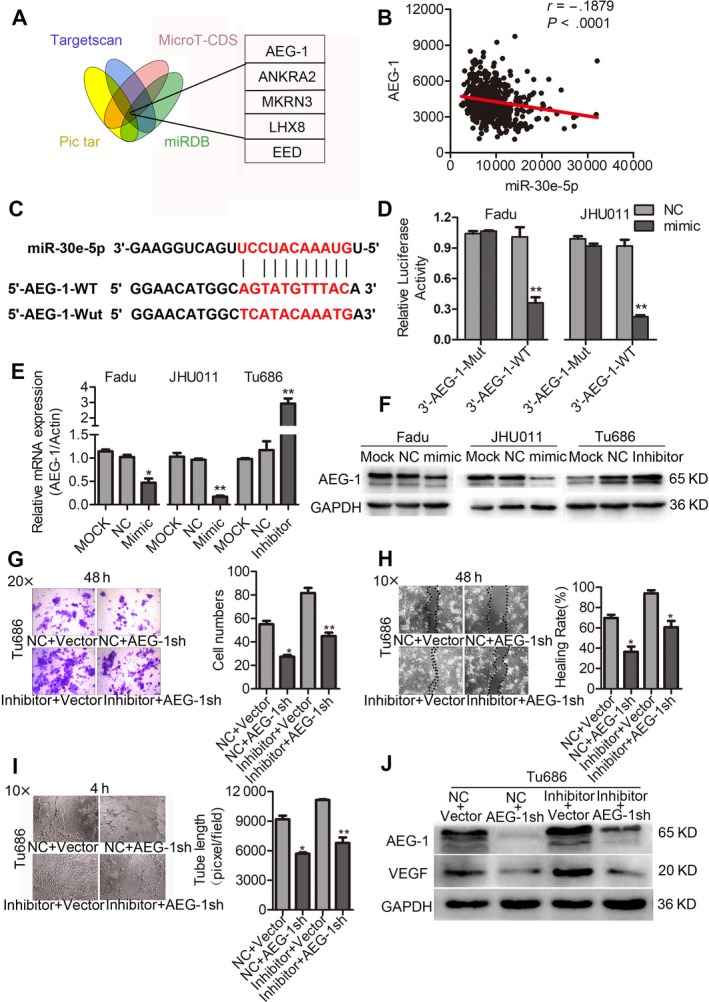
AEG‐1 is a direct target of miR‐30e‐5p and rescues its suppressive effects in squamous cell carcinoma of head and neck (SCCHN). A, The prediction algorithms of TargetScanHuman, Pictar, miRBD and MicroT‐CDS were chosen to screen the potential mRNAs of miR‐30e‐5p. B, Correlations between expression of miR‐30e‐5p and AEG‐1 in TCGA databases. C, The putative binding sequence of miR‐30e‐5p in AEG‐1 3′‐UTR; mutations were generated as indicated. D, Luciferase reporter assays showed AEG‐1 was a direct binding target of miR‐30e‐5p. Relative luciferase activity is presented as the ratio of Firefly/Renilla luciferase activity. E and F, Relative expression of AEG‐1 mRNA (E) and protein (F) in Fadu and JHU011 cells that transfected with miR‐30e‐5p mimic, and in Tu686 cells transfected with miR‐30e‐5p inhibitor. We calculated the relative mRNA expression using the 2−ΔΔCT method. G‐J, Effects of restoration of AEG‐1 on metastasis and angiogenesis in Tu686 cells determined by transwell migration (G), wound‐healing (H), endothelial cell tube formation assay (I) and western blot analysis (J). *P < 0.05; **P < 0.01
Subsequently, rescue experiments were performed to confirm the functional involvement of AEG‐1 in miR‐30e‐5p‐mediated SCCHN angiogenesis and metastasis. Tu686 cells were transfected with miR‐30e‐5p inhibitor and AEG‐1sh or corresponding empty vectors. Our data revealed that knockdown of AEG‐1 mitigated the repression of migration, invasion and angiogenesis in Tu686 cells (Figure 6G‐I). Similar to the phenotypical changes in the above rescue experiments, AEG‐1 restored the expression of proangiogenic factor VEGF (Figure 6J). Therefore, our data clearly suggest that AEG‐1 is involved in miR‐30e‐5p‐mediated angiogenesis and metastasis.
4. DISCUSSION
In this study, we found that miR‐30e‐5p was correlated with the aggressive characteristics in patients with SCCHN, which was an independent factor for prognosis in patients with SCCHN. A series of in vitro and in vivo experiments further identified that miR‐30e‐5p impeded the invasion and metastasis of SCCHN via the modulation of both EMT occurrence and cancer angiogenesis. Our study provides solid evidence demonstrating that miR‐30e‐5p is a valuable prognosis biomarker and a critical inhibitor for SCCHN metastasis.
The clinical significance of miR‐30e‐5p, especially its prognostic value in SCCHN, was a key highlight of our current investigation. As a metastasis suppressor, miR‐30e‐5p has been reported to be downregulated in several malignant carcinomas, such as bladder cancer,28 colorectal cancer,29 nasopharyngeal carcinoma30 and head and neck cancer.31 In the available clinical studies based on tumor samples, low expression of miR‐30e‐5p always exists in patients with more aggressive clinical characteristics, including advanced TNM stages and metastasis, which is consistent with our conclusion. However, Saleh et al determined that the miR‐30 family, including miR‐30a‐5p, was an important tumor suppressor; our study mainly sought to elucidate the metastasis regulator of miR‐30e‐5p.31 Our survival and Cox regression analyses based on the expression level of miR‐30e‐5p strengthen the notion that miR‐30e‐5p is a valuable metastasis biomarker with predictive potential in patients with SCCHN, which also needs to be tested in other solid carcinomas with large patient numbers to broaden its clinical significance.
Metastasis is a critical malignant behavior for SCCHN cells, which tremendously influences the treatment strategy decisions and final prognosis of patients, including SCCHN.2 Consistent with the clinical results, loss and gain of functional experiments clearly indicated the critical role of miR‐30e‐5p in SCCHN metastasis. Several studies have confirmed that miR‐30e‐5p is a microRNA that is closely associated with metastasis in vitro28, 29, 30; however, how miR‐30e‐5p blocks cancer metastasis and its exact molecular mechanisms remain largely unknown. In the process of metastasis, metastatic cancer cells are required to detach the primary lesion and enhance the migratory and invasive abilities, in which EMT is indispensable in the initiation of metastasis.5 Our data clearly demonstrated that miR‐30e‐5p, as an endogenous metastasis suppressor, together with its target AEG‐1, inhibited EMT and then impeded SCCHN metastasis. Only Ma et al have reported that MiR‐30e‐5p regulates the process of EMT in vitro30; however, we also provide more pervasive in vivo evidence to strengthen the links between miR‐30e‐5p and EMT. In addition, mounting evidence has indicated that AEG‐1, as an oncogene, has an important function in the process of EMT and metastasis in diverse human solid cancers.32, 33, 34 miR‐30e‐5p and AEG‐1 can form an miR‐30e‐5p/AEG‐1 axis pathway, which regulates the metastasis and EMT of SCCHN.
Angiogenesis is an essential step for cancer progression, local invasion and distant dissemination; therefore, it plays a very important role in cancer metastasis.35 A number of anti–angiogenesis agents have been approved by the FDA and are being used in cancer treatment.36 Our study, for the first time, confirmed the critical role of miR‐30e‐5p/AEG‐1 in cancer angiogenesis. In our study, data from coculture system initially revealed that miR‐30e‐5p repressed the migration of blood vessel epithelial cells (HUVEC), indicating the potential role of miR‐30e‐5p in cancer angiogenesis. Therefore, in vivo Matrigel angiogenesis plug and chick chorioallantoic membrane vascular assays were also used to determine its role in angiogenesis. As expected, both experimental models verified that miR‐30e‐5p overexpression dramatically reduced the microvessel density in cancer angiogenesis, which suggests the pivotal function of miR‐30e‐5p in the regulation of SCCHN angiogenesis.
To decipher the molecular mechanisms, a panel of cytokines and chemokines associated with cancer angiogenesis were quantified. Our data showed that miR‐30e‐5p overexpression obviously reduced the expression of multiple proangiogenic factors, including VEGF and HGF, both of which are the main stimulators for tumor blood vessel formation.9, 12, 27, 36 Apart from VEGF and HGF, IL‐8,37 FGF12 and CSF/1/2/338 are also potent proangiogenic factors, which were correspondingly decreased after miR‐30e‐5p overexpression. In addition, as a direct target of miR‐30e‐5p, AEG‐1 overexpression restored the level of part of the proangiogenic factors, revealing that AEG‐1, indeed, participated in miR‐30e‐5p‐mediated angiogenesis. However, some other proangiogenic factors, including FGF, were not changed following AEG‐1 overexpression, which suggests that other potential signaling pathways may be affected by miR‐30e‐5p.
Micro RNA regulate the malignant behaviors of cancer cells by controlling distinct gene expression, including that of oncogenes and/or suppressors.39 Specific miRNA synchronously inhibits multiple target genes by directly binding to the 3′‐UTR or 5′‐UTR region of mRNA.40 Based on review of the literature (present study), miR‐30e‐5p modulates diverse mRNA targets in different disease situations, including cancer. These target genes are listed as follows: NFAT5,41 TUSC3,42 Bim,43 USP22,30 MYBL2,44 ITGA6,29 ITGB1,29 LRP6 45 and YAP1 45. In our current study, miR‐30e‐5p was found to directly link to the 3′‐UTR domain of AEG‐1 mRNA, which accelerates the degradation of AEG‐1 mRNA and eventually reduces the level of AEG‐1 protein. In addition, TCGA data analysis revealed that miR‐30e‐5p and AEG‐1 mRNA display an opposite expression status in SCCHN samples. Taken together, these data reveal that AEG‐1 is a direct downstream target of miR‐3e‐5p. AEG‐1, as a multifunctional oncogene, have been reported to play critical roles in several cancer biological malignant behaviors, including transformation, metastasis, angiogenesis and therapeutic resistance.46 More importantly, restoration of AEG‐1 level abolished the enhancement of metastasis capability caused by miR‐30e‐5p downexpression, which suggests that AEG‐1 is involved in the effects mediated by miR‐30e‐5p, although we have to acknowledge that other potential targets of miR‐30e‐5p may also participate in this process.
In summary, our current data demonstrated that decreased expression of miR‐30e‐5p was significantly correlated with the malignant traits of SCCHN, particularly metastasis. Low expression of miR‐30e‐5p suggests a poor prognosis in patients with SCCHN. Most importantly, in vitro and in vivo evidence revealed that miR‐30e‐5p directly targeted oncogene AEG‐1, and repressed the invasion and metastasis by blocking EMT and angiogenesis. Therefore, miR‐30e‐5p and AEG‐1 represent promising molecular treatment targets to conquer metastatic SCCHN cancer.
DISCLOSURE
This study has no potential conflict of interest to declare.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Nos. 81874133, 81773243, 81772903, 81602684 and 81974424), the National Key Research and Development Program of China (2016YFC0902003), the Natural Science Foundation of Hunan Province (Nos. 2019JJ40481, 2019JJ50944, 2018JJ2630 and 2017JJ3488), the Huxiang Young Talent Project (No. 2018RS3024), the Xiangya Hospital‐Sinobioway Clinic and Rehabilitation Research Foundation (No. XYWM2015123) and the Fundamental Research Funds for the Central South University (No. 1053320182447).
Zhang S, Li G, Liu C, et al. miR‐30e‐5p represses angiogenesis and metastasis by directly targeting AEG‐1 in squamous cell carcinoma of the head and neck. Cancer Sci. 2020;111:356–368. 10.1111/cas.14259
Shuiting Zhang and Guo Li contributed equally to this paper.
Contributor Information
Yuanzheng Qiu, Email: xyqyz@hotmail.com.
Yong Liu, Email: liuyongent@csu.edu.cn.
REFERENCES
- 1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137‐2150. [DOI] [PubMed] [Google Scholar]
- 2. Allen CT, Law JH, Dunn GP, Uppaluri R. Emerging insights into head and neck cancer metastasis. Head Neck. 2013;35:1669‐1678. [DOI] [PubMed] [Google Scholar]
- 3. Elsheikh MN, Rinaldo A, Hamakawa H, et al. Importance of molecular analysis in detecting cervical lymph node metastasis in head and neck squamous cell carcinoma. Head Neck. 2006;28:842‐849. [DOI] [PubMed] [Google Scholar]
- 4. Cai GM, Huang DH, Dai YZ, et al. Analysis of transcriptional factors and regulation networks in laryngeal squamous cell carcinoma patients with lymph node metastasis. J Proteome Res. 2012;11:1100‐1107. [DOI] [PubMed] [Google Scholar]
- 5. Kalluri R, Weinberg RA. The basics of epithelial‐mesenchymal transition. J Clin Invest. 2009;119:1420‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395‐412. [DOI] [PubMed] [Google Scholar]
- 7. Wellenstein MD, de Visser KE. Cancer‐cell‐intrinsic mechanisms shaping the tumor immune landscape. Immunity. 2018;48:399‐416. [DOI] [PubMed] [Google Scholar]
- 8. Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18:139‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249‐257. [DOI] [PubMed] [Google Scholar]
- 10. Loizzi V, Del Vecchio V, Gargano G, et al. Biological pathways involved in tumor angiogenesis and bevacizumab based anti–angiogenic therapy with special references to ovarian cancer. Int J Mol Sci. 2017;18:1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yadav L, Puri N, Rastogi V, Satpute P, Sharma V. Tumour angiogenesis and angiogenic inhibitors. A review. J Clin Diagn Res. 2015;9:XE01‐XE5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vasudev NS, Reynolds AR. Anti–angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460‐469. [DOI] [PubMed] [Google Scholar]
- 14. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Domingues C, Serambeque BP, Laranjo Candido MS, et al. Epithelial‐mesenchymal transition and microRNAs: challenges and future perspectives in oral cancer. Head Neck. 2018;40:2304‐2313. [DOI] [PubMed] [Google Scholar]
- 17. Koshizuka K, Hanazawa T, Arai T, Okato A, Kikkawa N, Seki N. Involvement of aberrantly expressed microRNAs in the pathogenesis of head and neck squamous cell carcinoma. Cancer Metastasis Rev. 2017;36:525‐545. [DOI] [PubMed] [Google Scholar]
- 18. Liu G, Zheng J, Zhuang L, et al. A Prognostic 5‐lncRNA expression signature for head and neck squamous cell carcinoma. Sci Rep. 2018;8:15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu C, Wang Y, Li G, et al. LncRNA PVT1 promotes malignant progression in squamous cell carcinoma of the head and neck. J Cancer. 2018;9:3593‐3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan H, Zhu G, She L, et al. MiR‐98 inhibits malignant progression via targeting MTDH in squamous cell carcinoma of the head and neck. Am J Cancer Res. 2017;7:2554‐2565. [PMC free article] [PubMed] [Google Scholar]
- 21. Schmittgen TD, Livak KJ. Analyzing real‐time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101‐1108. [DOI] [PubMed] [Google Scholar]
- 22. Jing Q, Li G, Chen X, et al. Wnt3a promotes radioresistance via autophagy in squamous cell carcinoma of the head and neck. J Cell Mol Med. 2019;23:4711‐4722.31111621 [Google Scholar]
- 23. Qin Y, Wang J, Zhu G, et al. CCL18 promotes the metastasis of squamous cell carcinoma of the head and neck through MTDH‐NF‐kappaB signalling pathway. J Cell Mol Med. 2019;23:2689‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang D, Qiu Y, Li G, et al. KDM5B overexpression predicts a poor prognosis in patients with squamous cell carcinoma of the head and neck. J Cancer. 2018;9:198‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Su Z, Li G, et al. Increased expression of metadherin protein predicts worse disease‐free and overall survival in laryngeal squamous cell carcinoma. Int J Cancer. 2013;133:671‐679. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Xie C, Zhang X, et al. Elevated expression of HMGB1 in squamous‐cell carcinoma of the head and neck and its clinical significance. Eur J Cancer. 2010;46:3007‐3015. [DOI] [PubMed] [Google Scholar]
- 27. Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin. 2010;60:222‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Z, Qin H, Jiang B, et al. miR‐30e‐5p suppresses cell proliferation and migration in bladder cancer through regulating metadherin. J Cell Biochem. 2019;120:15924‐15932. [DOI] [PubMed] [Google Scholar]
- 29. Laudato S, Patil N, Abba ML, et al. P53‐induced miR‐30e‐5p inhibits colorectal cancer invasion and metastasis by targeting ITGA6 and ITGB1. Int J Cancer. 2017;141:1879‐1890. [DOI] [PubMed] [Google Scholar]
- 30. Ma YX, Zhang H, Li XH, Liu YH. MiR‐30e‐5p inhibits proliferation and metastasis of nasopharyngeal carcinoma cells by target‐ing USP22. Eur Rev Med Pharmacol Sci. 2018;22:6342‐6349. [DOI] [PubMed] [Google Scholar]
- 31. Saleh AD, Cheng H, Martin SE, et al. Integrated genomic and functional microRNA analysis identifies miR‐30‐5p as a tumor suppressor and potential therapeutic nanomedicine in head and neck cancer. Clin Cancer Res. 2019;25:2860‐2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He W, He S, Wang Z, et al. Astrocyte elevated gene‐1(AEG‐1) induces epithelial‐mesenchymal transition in lung cancer through activating Wnt/beta‐catenin signaling. BMC Cancer. 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srivastava J, Siddiq A, Gredler R, et al. Astrocyte elevated gene‐1 and c‐Myc cooperate to promote hepatocarcinogenesis in mice. Hepatology. 2015;61:915‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Y, Wang T, Sun Y, Sun W, Wang X. Astrocyte elevated gene‐1 promotes tumour growth and invasion by inducing EMT in oral squamous cell carcinoma. Sci Rep. 2017;7:15447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Medina J, Caveda L, Sanz‐Cameno P, et al. Hepatocyte growth factor activates endothelial proangiogenic mechanisms relevant in chronic hepatitis C‐associated neoangiogenesis. J Hepatol. 2003;38:660‐667. [DOI] [PubMed] [Google Scholar]
- 37. Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038‐6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Z, Malhotra PS, Thomas GR, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369‐1379. [PubMed] [Google Scholar]
- 39. Cheng CJ, Bahal R, Babar IA, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schirle NT, Sheu‐Gruttadauria J, MacRae IJ. Structural basis for microRNA targeting. Science. 2014;346:608‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qin X, Li C, Guo T, et al. Upregulation of DARS2 by HBV promotes hepatocarcinogenesis through the miR‐30e‐5p/MAPK/NFAT5 pathway. J Exp Clin Cancer Res. 2017;36:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu K, Xie F, Gao A, et al. SOX2 regulates multiple malignant processes of breast cancer development through the SOX2/miR‐181a‐5p, miR‐30e‐5p/TUSC3 axis. Mol Cancer. 2017;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mo B, Wu X, Wang X, Xie J, Ye Z, Li L. miR‐30e‐5p mitigates hypoxia‐induced apoptosis in human stem cell‐derived cardiomyocytes by suppressing bim. Int J Biol Sci. 2019;15:1042‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang K, Fu G, Pan G, et al. Demethylzeylasteral inhibits glioma growth by regulating the miR‐30e‐5p/MYBL2 axis. Cell Death Dis. 2018;9:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen Z, Qiu H, Ma L, et al. miR‐30e‐5p and miR‐15a synergistically regulate fatty acid metabolism in goat mammary epithelial cells via LRP6 and YAP1. Int J Mol Sci. 2016;17:1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Srivastava J, Robertson CL, Rajasekaran D, et al. AEG‐1 regulates retinoid X receptor and inhibits retinoid signaling. Cancer Res. 2014;74:4364‐4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


