Figure 4.
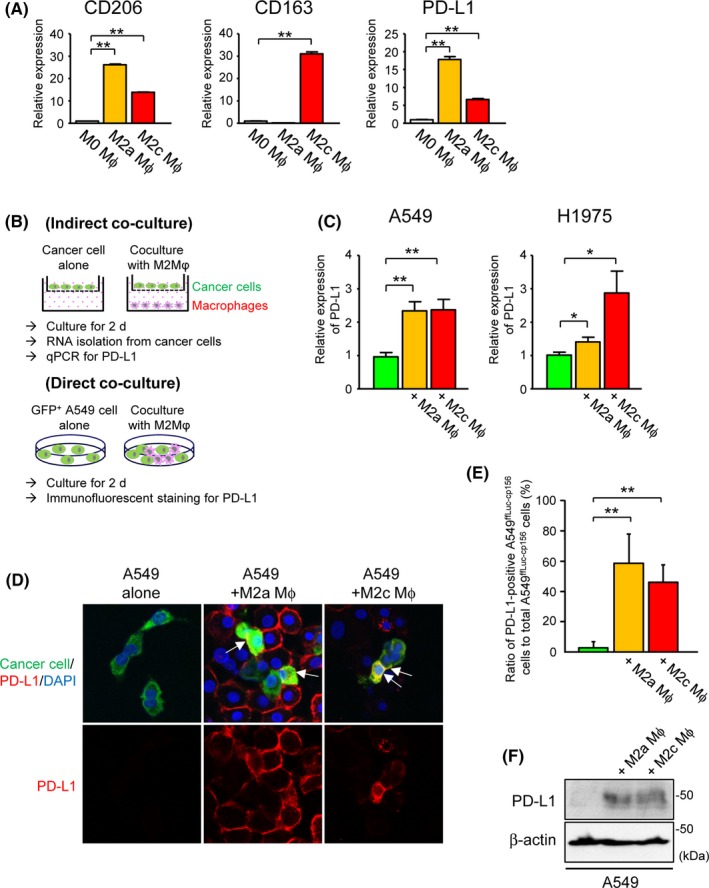
Upregulation of tumor programmed death‐ligand 1(PD‐L1) expression by co–culture with peripheral blood mononuclear cell (PBMC)‐derived M2‐differentiated macrophages. A, Relative expression of CD206, CD163 or PD‐L1 in human PBMC‐derived M0‐differentiated, M2a‐differentiated and M2c‐differentiated macrophages by real‐time quantitative PCR (qPCR) (n = 4). B, Description of co–culture schemes. For all experiments, human PBMC were differentiated into M2a or M2c macrophages prior to co–culture with human lung cancer cell lines. Following indirect or direct co–culture with these macrophages for 2 d, PD‐L1 expression in human lung cancer cell lines was evaluated by qPCR or immunofluorescent staining. C, Relative expression of PD‐L1 in A549 or H1975 cells indirectly co–cultured with human PBMC‐derived M2a‐differentiated or M2c‐differentiated macrophages by qPCR (n = 4). D, Representative images of immunofluorescent staining for PD‐L1 in Venus‐expressing A549ffLuc‐cp156 cells directly co–cultured with PBMC‐derived M2a‐differentiated or M2c‐differentiated macrophages. Arrows indicate A549ffLuc‐cp156 cells showing positive membrane staining for PD‐L1. E, Ratio of PD‐L1‐positive A549ffLuc‐cp156 cells to total A549ffLuc‐cp156 cells in the presence or absence of PBMC‐derived M2a‐ or M2c‐differentiated macrophages (n = 5). F, Representative immunoblot of PD‐L1 in A549 cells indirectly co–cultured with human PBMC‐derived M2a‐differentiated or M2c‐differentiated macrophages. β‐actin was used as a loading control. ∗ P < 0.05, ∗∗ P < 0.01
