Abstract
Nivolumab 3 mg/kg every 2 weeks (Q2W) has been approved in Japan for various cancers; however, use of a flat dose is expected to simplify dosing and administration. A quantitative clinical pharmacology approach was used to assess the benefit‐risk profile of nivolumab 240 mg Q2W relative to the approved dose of nivolumab 3 mg/kg Q2W in Japanese patients. Three exposure‐response safety analyses were performed for adverse events that led to discontinuation/death, were grade 3 or higher, and were immune‐mediated and grade 2 or higher for Japanese patients diagnosed with one of multiple tumor types. Exposure‐response analyses of efficacy were evaluated for overall survival and objective response rate. Exposures of nivolumab 240 mg Q2W were 37% higher than those of nivolumab 3 mg/kg Q2W in Japanese patients across the tumor types analyzed. Predicted safety profiles at the two doses differed by less than 2% across tumor types for adverse events leading to discontinuation/death, adverse events of grade 3 or higher, or immune‐mediated adverse events of grade 2 or higher. In addition, the predicted 1‐year and 2‐year overall survival rates, the mean overall survival and the objective response rates were comparable between the doses regardless of the tumor type analyzed. Overall, these results demonstrated that the benefit‐risk of nivolumab 240 mg Q2W was comparable to that of the previously approved 3 mg/kg Q2W dosing regimen, and was the basis for the approval of the 240 mg Q2W as an alternative dosing regimen for treatment in Japanese patients across multiple tumor types.
Keywords: cancer immunotherapy, clinical pharmacology, flat dosing, Japanese patients, nivolumab
Exposure‐response analyses of nivolumab in Japanese patients indicate comparable safety and efficacy outcomes for a flat dose of 240 mg every 2 weeks relative to the previously approved weight‐based dose of 3 mg/kg. These data suggest that a flat dose is a safe and effective alternative to the weight‐based dose in Japanese patients, across a range of tumor types.

1. INTRODUCTION
Nivolumab is a fully human IgG4 monoclonal antibody that binds programmed death 1 (PD‐1) on activated T cells to act as an antagonist and potentiate T‐cell responses.1 The first global approval for nivolumab was in Japan in 2014 for the treatment of unresectable melanoma.2 Nivolumab is now approved for first‐line treatment of patients with unresectable or metastatic melanoma (as monotherapy or in combination with ipilimumab) and as a second‐line agent for patients with metastatic non–small cell lung cancer (NSCLC), advanced renal cell carcinoma (RCC), classical Hodgkin lymphoma (cHL), squamous cell carcinoma of the head and neck (SCCHN), and urothelial cancer (UC) in the United States and the European Union, as well as for metastatic colorectal cancer and hepatocellular carcinoma in the United States.3 In addition, nivolumab was recently approved as an adjuvant treatment for patients with completely resected melanoma in the United States.3
Nivolumab was initially approved at a weight‐based dose of 2 mg/kg every 3 weeks in Japan2 and 3 mg/kg every 2 weeks (Q2W) in the United States and the European Union.4 Use of nivolumab at 3 mg/kg Q2W in Japan was approved after its efficacy and safety were demonstrated in Japanese patients.5 Evaluation of pharmacokinetics in patients with solid tumors has shown that 1, 3 and 10 mg/kg doses of nivolumab result in somewhat higher exposure (reflected by maximum plasma concentration and area under the concentration‐time curve) in Japanese and Korean patients versus those from the United States but that these small differences would not be expected to have an impact on efficacy or safety.6
Investigations into exposure levels for monoclonal antibody therapy found that most of these antibody treatments demonstrate relatively flat dose‐response relationships,7, 8, 9, 10 suggesting that a body‐weight‐based regimen may not be necessary. It has also been shown that body‐weight‐based dosing does not always offer an advantage over flat dosing for decreasing exposure variability and that the pharmacokinetic variability from either a flat‐dose or a body‐weight regimen is moderate when considering resulting pharmacodynamics, efficacy and safety.11 Specifically, an exposure‐response (E‐R) analysis of nivolumab in previously untreated patients with advanced melanoma reported that the time‐averaged concentration after the first dose of nivolumab is not a significant predictor of overall survival (OS) in patients with advanced melanoma treated with doses ranging between 0.1 and 10 mg/kg every 2 weeks.12 Similar results have been reported from E‐R analyses of data from patients with NSCLC, where no significant association between nivolumab exposure and OS or toxicity was found.10
A potential benefit of flat dosing is simplified administration of a drug across a wide range of tumor types, providing greater convenience to healthcare providers by helping to facilitate dosing calculations and drug preparation, improving patient compliance, and possibly helping to reduce healthcare costs.4, 9, 13, 14 In addition, preparation of a body‐weight dose may result in excess drugs being prepared, which could be avoided with a flat dose. Lack of excess drug will help reduce both the waste of product and the potential for inappropriate use of prepared medicine between patients.14 For example, improper use of a single prepared medication vial has been associated with infection events and outbreaks in the outpatient setting.16 Of 26 infection outbreaks that occurred due to unsafe injection practices in healthcare facilities, 73% were associated with sharing a single prepared vial with more than one patient.15
Given that nivolumab has linear PK over a dose range of 0.1 to 10 mg/kg across multiple tumor types, the 240 mg Q2W regimen has been proposed based on the approximate median body weight of 80 kg for subjects treated in nivolumab clinical trials (N = 3458).4 Most phase 3 clinical trials for multiple tumor types are currently conducted with 240 mg flat dose. ICH E17 states that the dose regimens in confirmatory multi‐regional clinical trials should in principle be the same in all participating ethnic population unless earlier trial data show a clear difference in dose‐response and/or exposure‐response relationships for an ethnic population.17 Based on a demonstration of the similarity of predicted exposure and efficacy/safety responses in population pharmacokinetic (PPK) and E‐R analyses,4, 13 a 240 mg flat dose is selected and investigated for a Japanese population as well as a non–Japanese population in accordance with ICH E17.
Data from both global and regional Japanese studies were used to conduct the E‐R analyses presented here to characterize the relationship between nivolumab exposure and its efficacy and safety in the Japanese population and to assess the potential impact of changing from a 3 mg/kg Q2W dose to a 240 mg Q2W dose. Efficacy outputs were generated for patients diagnosed with advanced melanoma, squamous (SQ) or non–squamous (NSQ) NSCLC, or RCC, and safety outputs were generated for the total Japanese population across a range of tumor types. Specifically, safety, OS, and objective response rate (ORR) of nivolumab at the flat dose of 240 mg Q2W were compared to those of the 3 mg/kg Q2W body‐weight‐normalized dose in global and Japanese patient populations.
2. MATERIALS AND METHODS
2.1. Patient population
Data from 10 global studies and 5 regional studies (ONO‐4538‐01, ONO‐4538‐02, ONO‐4538‐05, ONO‐4538‐06 and ONO‐4538‐08) of Japanese patients with various cancers (eg, melanoma, NSCLC, RCC, colorectal cancer, cHL, UC and SCCHN) were used in the safety and efficacy analyses described here. Doses for the analyses ranged from 1 to 10 mg/kg. Study descriptions and study numbers, number of Japanese patients and analysis type are all displayed in Table S1.
2.2. Pharmacokinetic model
A previously developed PPK mode12 was used to determine nivolumab exposure. The PPK model consisted of a linear, two‐compartment pharmacokinetic model with zero‐order intravenous infusion and first‐order elimination with time‐varying clearance. In the PPK model, 3939 patients (including 420 Japanese patients) were involved in PPK modeling and simulations. All exposure metrics (including Cavgd28, Cmind28, Cmin1, Cmax1, Cavg1, Cavgss, Cminss and Cmaxss) were determined from maximum a posteriori estimates of individual pharmacokinetic parameters after a flat dose and weight‐based dosing. The E‐R analyses used log‐transformed Cavgd28 (time‐averaged concentration of nivolumab over the first 28 days, or two doses administered Q2W) as the exposure measure. This measure was selected to avoid potentially confounding the E‐R analysis by changes in exposure due to time‐varying clearance, which has been shown to be associated with an efficacy response.18 Cavgd28 was log‐transformed because it spanned more than a 10‐fold range.
2.3. Exposure‐response analysis of safety
Three safety endpoints were selected to investigate a broad spectrum of clinically relevant adverse events (AE) and any potential differences between the two doses in global and Japanese studies (Table S1): AE that led to discontinuation (excluding those due to disease progression) or death (AE‐DC/D); grade 3 or higher AE (AE‐Grade 3+); and grade 2 or higher immune‐mediated AE (AE‐IM Grade 2+). A logistic regression model was developed using data from 2560 global patients, which was updated to include data from 273 Japanese patients to predict safety outcomes. In this model, the probability that patient i will experience an AE is given by:
where Xi represents the predictor variables and β o and β are the estimated parameters of the model.
2.4. Exposure‐response analysis of efficacy
Both OS and ORR were used as efficacy endpoints to assess and compare predicted efficacy of nivolumab 240 mg Q2W and 3 mg/kg Q2W in the noted global and Japanese studies (Table S1). Separate models for OS and ORR were developed from studies of patients diagnosed with melanoma, NSCLC (SQ and NSQ) or RCC. E‐R models of OS and ORR that included 1749 and 1710 patients, respectively, now included 134 Japanese patients from regional studies. Relationships between nivolumab exposure and OS and ORR, adjusted for previously identified covariate (ie, sex, age, body weight, region, performance status, risk score, prior treatment, programmed death ligand 1 status, tumor status/stage, M‐stage, baseline cell counts, lactate dehydrogenase [LDH] level and baseline clearance) effects, were described by a Cox proportional hazards model and a logistic regression model, respectively. The probability of achieving objective response (ORes) was described by a logistic regression model similar to that used to describe the probability of experiencing a safety event. The hazard of death of a particular patient (i) in the Cox proportional hazards model of OS is given by:
with ho(t) as the baseline hazard function, Xi the vector of predictor variables and β the vector of coefficients.
Individual survival probabilities for each patient were averaged to obtain a predicted OS curve, and mean survival probabilities at 1 and 2 years for each dose were also predicted. The predicted response rates for each dose were compared with that of control arms (ie, standard of care).
After model qualification by a visual predictive check, the models were used to predict hazard and odds ratios (200 and 1000 times, respectively, for OS and ORR) for each dose regimen. The median values and 95% confidence intervals (CI) were summarized and compared.
2.5. Safety and efficacy predictor
Various predictor variables (ie, body weight, age, sex, performance score, line of therapy and tumor type baseline clearance), in addition to nivolumab exposure, were assessed to estimate whether each would have an impact on the safety or efficacy of nivolumab treatment. An increased or decreased risk was determined based on hazard ratios. If a 95% CI range included 1.0, then the associated variable was not considered a significant prognostic factor for safety or efficacy. For example, a hazard ratio <1.0 for body weight and a CI range that does not include 1.0 would suggest a significantly increased risk for patients with a lower body weight. The variables assessed included log‐transformed Cavgd28, multiple baseline characteristics, prior treatment and tumor type (safety only). Ethnicity (Japanese vs non–Japanese) was evaluated as a covariate for melanoma and NSCLC (SQ and NSQ) in the efficacy analysis.
3. RESULTS
3.1. Comparisons of nivolumab exposure
The geometric mean and median of nivolumab exposure for E‐R safety analyses (Table 1) were computed for 273 Japanese patients enrolled in 9 different studies (Table S1) using a previously described PPK model (see Materials and Methods) to compare predictions of safety and efficacy of nivolumab treatment at 240 mg Q2W and 3 mg/kg Q2W. The baseline body weight of Japanese patients ranged from 33 kg to 105 kg, with a median body weight of 57.3 kg (Figure S1). Overall, exposure was higher for the 240 mg Q2W dose compared with the 3 mg/kg Q2W dose; specifically, the geometric mean Cavgd28 was 37% higher. When exposure was assessed in the E‐R efficacy analysis across tumors in 134 Japanese patients, a similar trend of a 28% to 35% increase in mean exposure was observed for the flat dose relative to the body‐weight dose (Table S2).
Table 1.
Summary of exposure in Japanese patients for the exposure‐response safety analysis
| Summary exposure, µg/mL | Geometric mean (CV%) | % Difference | Median (P05, P95) | ||
|---|---|---|---|---|---|
| 240 mg Q2W | 3 mg/kg Q2W | 240 mg Q2W | 3 mg/kg Q2W | ||
| Cavgd28 | 44.6 (21.4) | 32.5 (20.6) | 37.2 | 44.8 (31.6, 61.7) | 32.8 (21.9, 45.2) |
| Cmind28 | 38.4 (27.7) | 27.9 (25.8) | 37.6 | 38.9 (23.5, 59.5) | 29.3 (16.5, 41.3) |
| Cmax1 | 76.1 (21.0) | 55.3 (18.3) | 37.6 | 75.4 (54.6, 108.0) | 55.0 (40.7, 73.4) |
Cavgd28, nivolumab concentration over the first 28 d; Cmax1, peak concentration after the first dose; Cmind28, trough concentration at day 28; CV, coefficient of variation; P05, fifth percentile; P95, 95th percentile; Q2W, every 2 wk.
3.2. Exposure‐response analysis of safety
Previous E‐R models indicate that there is a flat exposure‐safety relationship across tumor types from dose levels ranging from 0.1 mg/kg to 10 mg/kg. In this study, the E‐R safety model predicted that, relative to nivolumab 3 mg/kg Q2W, the slightly higher range of Cavgd28 produced by 240 mg Q2W in Japanese patients may result in either no change or a negligible increase (≤2% difference, not statistically significant) in the proportion of patients who may have an AE‐DC/D, AE‐Grade 3+ or AE‐IM Grade 2 + for all tumor types (Figure 1). As demonstrated in the summary of each safety endpoint, the predicted proportion of AE was comparable for all tumor types assessed.
Figure 1.
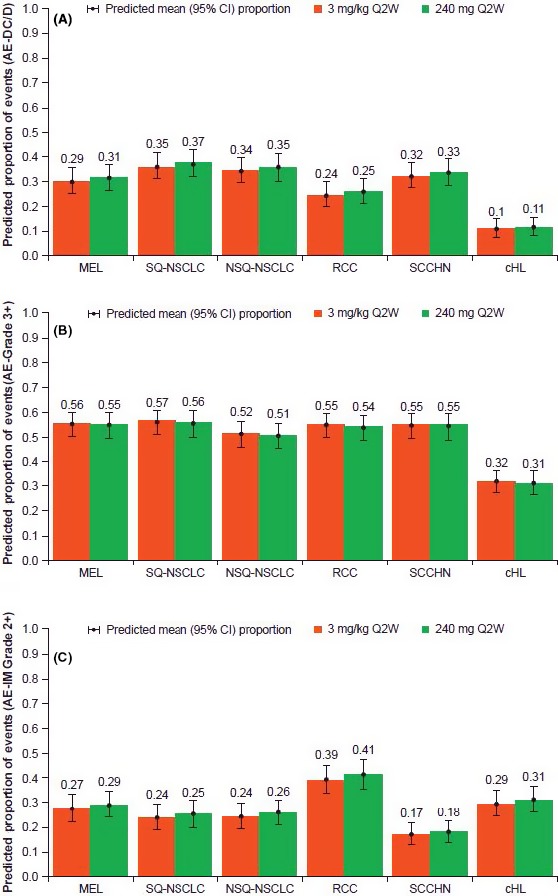
The proportion of adverse events (AE) were predicted based on the 240 mg Q2W or 3 mg/kg Q2W dosing and tumor type. A, Predicted proportion of AE leading to discontinuation or death. B, Predicted proportion of ≥grade 3 AE. C, Predicted proportion of >grade 2 immune‐mediated AE. AE‐DC/D, AE that lead to discontinuation (excluding those due to disease progression) or death; AE‐Grade 3+, AEs ≥grade 3; AE‐IM Grade 2+, immune‐mediated AE ≥grade 2; cHL, classical Hodgkin lymphoma; CI, confidence interval; MEL, melanoma; NSCLC, non–small cell lung cancer; NSQ, non–squamous; Q2W, every 2 wk; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; SQ, squamous
Baseline characteristics, line of therapy, nivolumab dose (via Cavgd28) and tumor type were analyzed to determine any effect of these variables on safety by estimating the risk of AE‐DC/D, AE‐Grade 3+ and AE‐IM Grade 2 + occurrence for the total study populations, including Japanese patients (Table S3). The odds ratios for nivolumab Cavgd28 to cause either an AE‐DC/D or AE‐Grade 3+ were 1.23 and 0.91, respectively. The 95% CI for these ratios included 1, indicating that increasing the nivolumab dose did not significantly impact the occurrence of AE‐DC/D or AE‐Grade 3+ in the tumor types assessed here. A slightly higher risk for AE‐IM Grade 2+ in patients with a higher nivolumab exposure (odds ratio 1.01; 95% CI 1.00, 1.01) was predicted, which may be due to the interaction of nivolumab with the immune system (discussed further below). Lower body weight was mildly associated with an elevated risk of developing an AE‐DC/D (odds ratio 0.98; 95% CI 0.97, 0.98). Having a performance score >0, more than one line of previous therapy and an increased clearance level were all identified as factors associated with a significantly increased chance of developing an AE‐DC/D or an AE‐Grade 3+.
3.3. Exposure‐response analysis of efficacy
Prediction of mean 1‐year and 2‐year OS probabilities based on the 240 mg Q2W or 3 mg/kg Q2W dosages is presented in Table 2. The mean OS for the proposed 240 mg Q2W regimen and the approved 3 mg/kg Q2W regimen was similar at both time points for melanoma (2‐year mean OS: 0.42 [95% CI 0.34, 0.57] and 0.47 [95% CI 0.42, 0.60], respectively) and the other tumor types. The predicted mean survival and 95% CI for 3 mg/kg Q2W and 240 mg Q2W highly overlapped in Japanese studies of patients with melanoma and NSCLC (SQ and NSQ) (Figure 2). ORR was also predicted and compared for the 240 mg Q2W and 3 mg/kg Q2W dosages in Japanese studies of patients with NSCLC and melanoma, and no differences were observed between doses within each tumor type assessed (Figure 3).
Table 2.
Predicted mean survival probabilities by dosage for studies including Japanese patients
| Tumor type (study) | Predicted mean survival probability (95% CI) | |||
|---|---|---|---|---|
| 3 mg/kg Q2W | 240 mg Q2W | |||
| 1 y | 2 y | 1 y | 2 y | |
|
Melanoma (CA209‐315/ONO‐4538‐08) |
0.67 (0.63, 0.77) |
0.47 (0.42, 0.60) |
0.62 (0.57, 0.75) |
0.42 (0.34, 0.57) |
|
SQ‐NSCLC (CA209‐131/ONO‐4538‐05) |
0.56 (0.50, 0.65) |
0.36 (0.30, 0.45) |
0.57 (0.52, 0.66) |
0.38 (0.33, 0.48) |
|
NSQ‐NSCLC (CA209‐132/ONO‐4538‐06) |
0.62 (0.57, 0.67) |
0.36 (0.31, 0.41) |
0.62 (0.57, 0.68) |
0.36 (0.32, 0.41) |
|
RCC (CA209‐025) |
0.76 (0.75, 0.78) |
0.53 (0.50, 0.55) |
0.76 (0.75, 0.78) |
0.53 (0.51, 0.55) |
CI, confidence interval; NSCLC, non–small cell lung cancer; NSQ, non–squamous; Q2W, every 2 wk; RCC, renal cell carcinoma, SQ, squamous.
Figure 2.
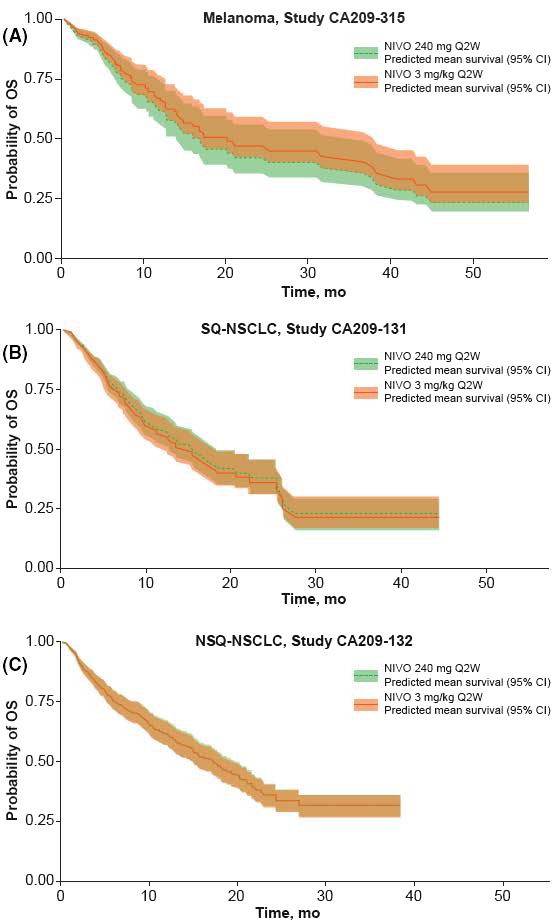
Predicted mean overall survival (OS) based on 240 mg Q2W or 3 mg/kg Q2W dosing in patients from Japanese studies in patients with the following tumor types: melanoma (A); squamous non–small cell lung cancer (SQ‐NSCLC) (B); non–squamous non–small cell lung cancer (NSQ‐NSCLC) (C). CI, confidence interval; NIVO, nivolumab; Q2W, every 2 wk
Figure 3.
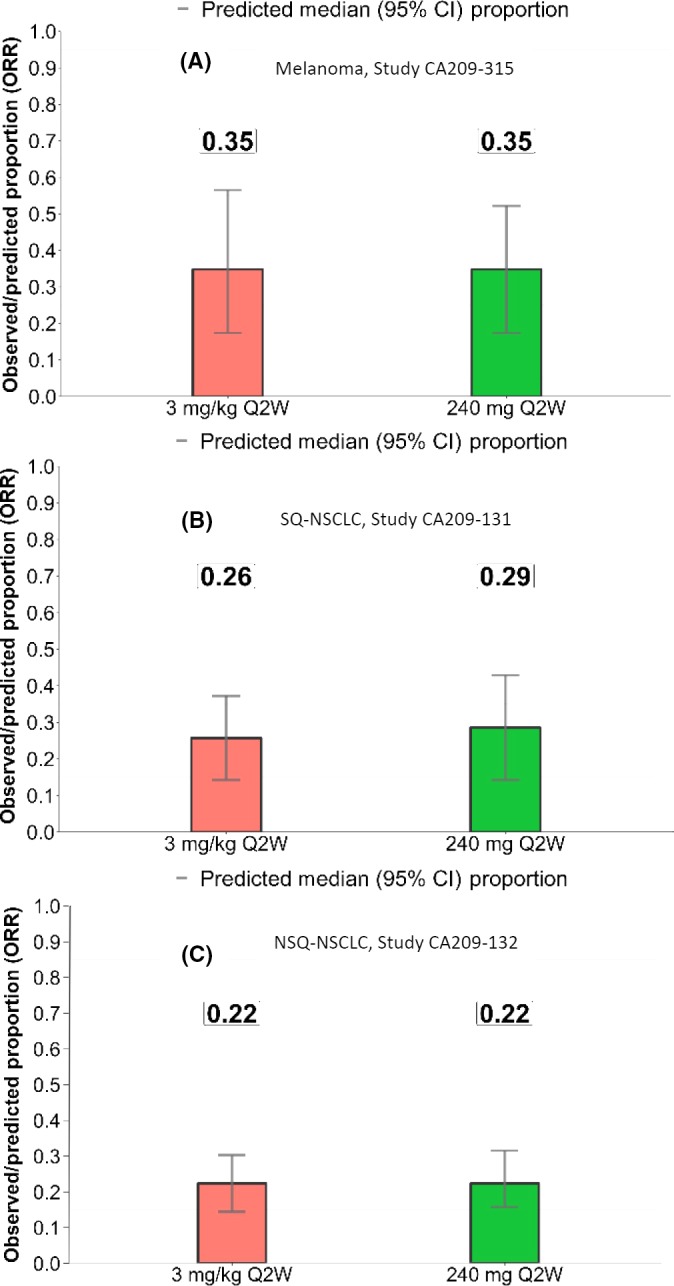
Predicted median objective response rate (ORR) based on 240 mg Q2W or 3 mg/kg Q2W dosing in patients from Japanese studies and with the following tumor types: melanoma (A); squamous non–small cell lung cancer (SQ‐NSCLC) (B); non–squamous non–small cell lung cancer (NSQ‐NSCLC) (C). Q2W, every 2 wk
Various baseline variables and demographic covariates, occurrence of prior treatment and Cavgd28 were analyzed across tumor types. Inclusion of variables previously identified as significantly associated with OS, based on dose, were included to enable an unbiased assessment of the E‐R relationship and to determine the impact of each variable on survival of study patients; outcomes of these analyses are reported in Table S4. Hazard ratios and 95% CI suggest a significantly increased risk of death for both lower body weight and higher baseline clearance level across all four tumor types assessed. Elevated LDH in patients with melanoma or NSCLC (SQ and NSQ) and a higher Cavgd28 in patients with melanoma were each predicted to significantly increase risk of death. In patients with NSQ‐NSCLC, a decreased risk of death was identified for Japanese versus non–Japanese patients.
Baseline and other variables were also assessed for relation to ORR for melanoma, RCC or NSCLC (SQ and NSQ) and are shown in Table S5. Baseline clearance significantly influenced ORR across each tumor type assessed. Body weight and sex were found to have a significant effect on ORR for patients with melanoma and RCC, respectively. Line of therapy, programmed death ligand 1 (PD‐L1) expression, smoking and body weight significantly influenced ORR in patients with NSQ‐NSCLC.
4. DISCUSSION
Recent pharmacokinetic and E‐R analyses have indicated that a flat dose of monoclonal antibody therapy to treat cancer has a benefit‐risk profile comparable to a body‐weight‐based regimen,4 which led to the approval for use of nivolumab 240 mg to treat melanoma, NSCLC (SQ and NSQ) and RCC in the United States3 and the European Union.19 The data reported in this analysis expand on recent investigations by including additional Japanese populations in the E‐R analyses, which can influence the flat‐dose strategy for approved indications in the Japanese market in addition to the new indication for treatment with nivolumab as monotherapy or combination therapy.
A higher exposure in Japanese patients is not surprising, as a 240 mg flat dose corresponds to a 3 mg/kg dose in patients weighing 80 kg, which was the approximate median body weight in the global studies in melanoma, NSCLC and RCC patients,4 and the Japanese patient population had an average body weight of approximately 60 kg. However, the higher exposure predicted for Japanese patients receiving 240 mg Q2W is well below exposures with 10 mg/kg Q2W,4 which has been reported to be a safe and tolerable dosing regimen across tumor types.19, 20 A flat dose of 240 mg was selected as a harmonized dose across all regions, including Japan, to facilitate global development of nivolumab monotherapy across tumor types. Although this dose produces slightly higher exposures in Japanese patients compared to the previously approved 3 mg/kg dose, the exposure‐response analyses presented in this manuscript demonstrate that the benefit‐risk of nivolumab remains unchanged. The higher exposures of nivolumab do not compromise either the safety or efficacy of nivolumab, given the flat exposure‐response relationships of efficacy and safety. The 240 mg Q2W dose is being investigated for tumor types and indications other than those for which nivolumab is currently approved in Japan, and the selection of a harmonized dose across all regions enables global clinical development in accordance with ICH E17.
There was no significant association (the 95% CI for the odds ratio included 1) between Cavgd28 of nivolumab and AE‐DC/D or AE‐Grade 3+. Body weight was significantly associated with AE‐DC/D (odds ratio 0.98 [95% CI 0.97, 0.98]). It should be noted, however, that body weight was not a significant predictor of AE‐IM Grade 2+, and the E‐R was also relatively flat for this measure of safety. AE‐IM is considered to be a more relevant measure of safety to assess the impact of a change in nivolumab dose than AE‐DC/D, as AE‐IM is more likely to be related to the mechanism of action of nivolumab.21, 22 Overall, the predicted safety profiles of nivolumab at 240 mg Q2W and 3 mg/kg Q2W were comparable, and the impact of flat dosing on AE risk was minimal.
In patients with melanoma, the risk of death seemed to be slightly higher with higher Cavgd28; however, there was overlap with regard to 95% CI in the predicted mean OS. The predicted mean 1‐year and 2‐year OS were quite similar for the flat and weight‐based doses, suggesting that a flat dose is unlikely to result in any clinically meaningful differences in efficacy. The increased risk of death for higher clearance, higher baseline LDH, and lower body weight in patients with melanoma23 or NSCLC10 observed in this analysis has been reported previously.
Results from the variable estimate analysis suggested that there may be a lower risk of death in Japanese versus non–Japanese patients for those diagnosed with NSQ‐NSCLC (95% CI 0.42, 0.99). It is worth noting that the percentage of patients who received subsequent therapy out of those who experienced disease progression or death is higher in the Japanese study (ONO‐4538‐06/CA209‐132; 83.6%) than in the global study (CA209‐057; 58.3%).
Unlike for melanoma and NSCLC, there was no regional Japanese study for patients with RCC; therefore, the model to predict ORR for RCC was not updated. The global phase 3 study (CA209‐025) for RCC, however, did enroll 37 Japanese patients, who were included in the datasets from the previous analyses. For this global RCC study, the predicted median nivolumab exposures (Cavgd28) for Japanese and total patients for 240 mg Q2W were 43.8 µg/mL and 33.9 µg/mL, respectively. Despite the approximately 37% higher predicted nivolumab exposure in Japanese patients compared with the overall population in the RCC study, the hazard ratio estimate for OS (mean 1.00 [range 0.90‐1.11]) and odds ratio estimate for ORR (mean 0.94 [range 0.78‐1.13]) were close to 1.0 and the 95% CI included 1. This suggests that efficacy would be similar between the Japanese and global populations.
Safety was assessed in a pooled group of tumor types; however, some other tumor types were not included in the efficacy E‐R analyses due to the lack of dose‐ranging data, and OS and ORR predictions were made for melanoma, NSCLC and RCC, but not SCCHN, UC or cHL. Because nivolumab acts by targeting the immune system instead of the tumor, and given that findings for the four tumor types investigated here are consistent, it is reasonable to speculate that other tumor types not evaluated in these analyses would also have flat E‐R relationships.
In conclusion, predicted safety and efficacy outcomes were comparable between the flat and weight‐based dose regimens in Japanese patients. A higher level of exposure to nivolumab in Japanese patients was predicted for 240 mg Q2W relative to 3 mg/kg Q2W; however, based on E‐R safety and efficacy analyses, the difference in exposure is not expected to significantly alter the safety or efficacy outcomes of nivolumab in treatment of Japanese patients. Overall, the results of these analyses demonstrated that the benefit–risk profile of nivolumab 240 mg Q2W was comparable to the previously approved nivolumab 3 mg/kg Q2W regimen. These results were the basis for the approval of nivolumab 240 mg Q2W for treatment in Japanese patients across multiple tumor types.
DISCLOSURE
Shinji Uemura and Tomoya Ohno are employees of ONO Pharmaceutical. Di Bei and Amit Roy are employees of Bristol‐Myers Squibb and Mayu Osawa is employee of Bristol‐Myers Squibb KK. Mayumi Hasegawa was employed by Bristol‐Myers Squibb KK at the time this work was completed. This study was designed and funded by Bristol‐Myers Squibb and ONO Pharmaceutical. Nivolumab supply and support to collect and analyze the data were provided by Bristol‐Myers Squibb. All authors had access to the complete data, significantly contributed to development of the manuscript and had final responsibility for the decision to submit the manuscript for publication.
Supporting information
ACKNOWLEDGMENTS
We thank the patients and their families for making the studies possible. Professional medical writing and editorial assistance were provided by Kelly M. Fahrbach, PhD, at StemScientific, funded by Bristol‐Myers Squibb KK and ONO Pharmaceutical. We would like to acknowledge the scientific contributions of Vijay Ivaturi, PhD, and Mathangi Gopalakrishnan, PhD, from University of Maryland, USA.
Bei D, Osawa M, Uemura S, et al. Benefit‐risk assessment of nivolumab 240 mg flat dose relative to 3 mg/kg Q2W regimen in Japanese patients with advanced cancers. Cancer Sci. 2020;111:528–535. 10.1111/cas.14252
These trials were registered at clinicaltrials.gov with registration numbers NCT00836888, NCT00730639, NCT01642004, NCT01673867, NCT01721759, NCT01668784, NCT02041533, NCT01721746, NCT01721772, NCT02105636, and NCT02387996; or at clinicaltrials.jp with registration numbers JapicCTI‐111681, JapicCTI‐132072, and JapicCTI‐132073.
Funding information
Bristol‐Myers Squibb.
DATA AVAILABILITY STATEMENT
The Bristol‐Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html .
REFERENCES
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. News EO .Nivolumab receives manufacturing and marketing approval in japan for the treatment of unresectable melanoma. July 10, 2014.
- 3. Opdivo (nivolumab) [package insert]. Princeton, NJ: Bristol‐Myers Squibb, 2018. [Google Scholar]
- 4. Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit‐risk profile of a 240‐mg flat dose relative to a 3‐mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28:2002‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamazaki N, Kiyohara Y, Uhara H, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: A phase II study. Cancer Sci. 2017;108:1223‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee KW, Lee DH, Kang JH, et al. Phase I pharmacokinetic study of nivolumab in Korean patients with advanced solid tumors. Oncologist. 2018;23:155‐e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size‐based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49:1012‐1024. [DOI] [PubMed] [Google Scholar]
- 8. Zhang S, Shi R, Li C, Parivar K, Wang DD. Fixed dosing versus body size‐based dosing of therapeutic peptides and proteins in adults. J Clin Pharmacol. 2012;52:18‐28. [DOI] [PubMed] [Google Scholar]
- 9. Hendrikx J, Haanen J, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22:1212‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng Y, Wang X, Bajaj G, et al. Nivolumab exposure‐response analyses of efficacy and safety in previously treated squamous or nonsquamous non–small cell lung cancer. Clin Cancer Res. 2017;23:5394‐5405. [DOI] [PubMed] [Google Scholar]
- 11. Bai S, Jorga K, Xin Y, et al. A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet. 2012;51:119‐135. [DOI] [PubMed] [Google Scholar]
- 12. Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model‐based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6:58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheng J, Srivastava S, Sanghavi K, et al. Clinical pharmacology considerations for the development of immune checkpoint inhibitors. J Clin Pharmacol. 2017;57(Suppl 10):S26‐S42. [DOI] [PubMed] [Google Scholar]
- 15. CDC. Protect patients against preventable harm from improper use of single‐dose/single‐use vials. Altanta, Georgia: CDC. https://www.cdc.gov/injectionsafety/cdcposition-singleusevial.html. Accessed August 30, 2016.
- 16. Centers for Disease Control and Prevention . Outbreaks and patient notifications in outpatient settings, selected examples, 2010-2014. https://www.cdc.gov/hai/settings/outpatient/outbreaks-patientnotifications.html. Accessed July 10, 2015.
- 17. ICH . General principles for planning and design of multi‐regional clinical trials E17 final version. ICH; https://ich.org.ich01.nine.ch/page/efficacy-guidelines#17. Accessed November 16, 2017. [Google Scholar]
- 18. Liu C, Yu J, Li H, et al. Association of time‐varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin Pharmacol Ther. 2017;101:657‐666. [DOI] [PubMed] [Google Scholar]
- 19. Agrawal S, Feng Y, Roy A, Kollia G, Lestini B. Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy. J Immunother Cancer. 2016;4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD‐1 antibody in cancer. N Engl J Med. 2012;366:2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baxi S, Yang A, Gennarelli RL, et al. Immune‐related adverse events for anti–PD‐1 and anti–PD‐L1 drugs: systematic review and meta‐analysis. BMJ. 2018;360:k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stucci S, Palmirotta R, Passarelli A, et al. Immune‐related adverse events during anticancer immunotherapy: Pathogenesis and management. Oncol Lett. 2017;14:5671‐5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Feng Y, Bajaj G, et al. Quantitative characterization of the exposure-response relationship for cancer immunotherapy: a case study of nivolumab in patients with advanced melanoma. CPT Pharmacometrics Syst Pharmacol. 2017;6:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Bristol‐Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html .


