Figure 1.
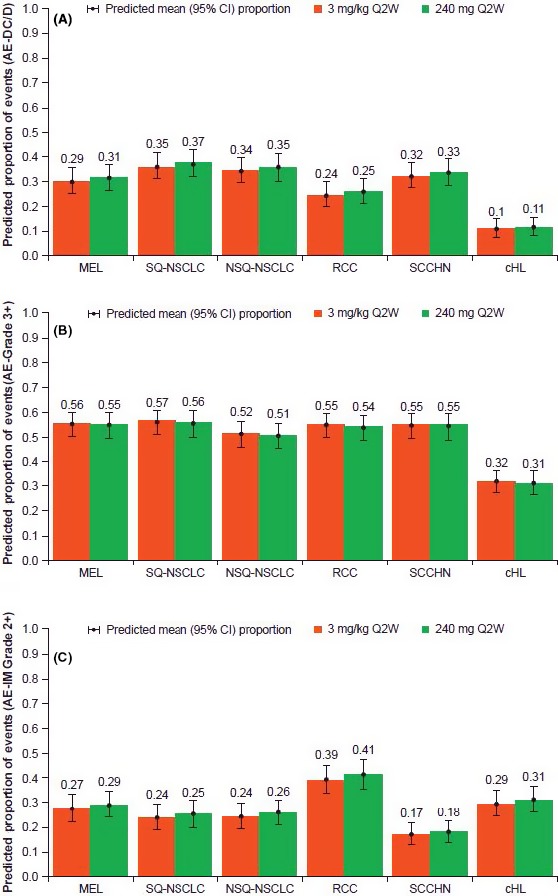
The proportion of adverse events (AE) were predicted based on the 240 mg Q2W or 3 mg/kg Q2W dosing and tumor type. A, Predicted proportion of AE leading to discontinuation or death. B, Predicted proportion of ≥grade 3 AE. C, Predicted proportion of >grade 2 immune‐mediated AE. AE‐DC/D, AE that lead to discontinuation (excluding those due to disease progression) or death; AE‐Grade 3+, AEs ≥grade 3; AE‐IM Grade 2+, immune‐mediated AE ≥grade 2; cHL, classical Hodgkin lymphoma; CI, confidence interval; MEL, melanoma; NSCLC, non–small cell lung cancer; NSQ, non–squamous; Q2W, every 2 wk; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; SQ, squamous
