Supplemental Digital Content is available in the text
Keywords: amyloidosis, SPECT/CT, 99mTc-DPD, whole body scintigraphy
Abstract
Although pathological confirmation is the gold standard for diagnosis of amyloidosis, there is a need for a relevant imaging modality to identify involved organs and evaluate disease extent. Thus, we prospectively investigated imaging findings of 99mTc-DPD scintigraphy in AL and ATTR amyloidosis.
A total of 21 subjects with pathologically confirmed AL or ATTR amyloidosis were included. Pretreatment whole body 99mTc-DPD planar scanning and regional SPECT/CT were performed in all subjects. For allegedly involved organs, 99mTc-DPD uptake was visually and semi-quantitatively evaluated on a 4-point scale (grade 0: no uptake, 1: uptake less than spine, 2: uptake similar to spine, and 3: uptake greater than spine).
There were 29 organs involved in AL and 12 in ATTR. Significant 99mTc-DPD uptake was found in 24 organs (sensitivity = 82.8%) in AL and 9 organs (sensitivity = 75.0%) in ATTR. Additional SPECT/CT was helpful to ensure abnormal DPD uptake in the involved organs, which was uncertain by attenuation in planar imaging. Degree of 99mTc-DPD uptake was significantly higher in ATTR compared with AL amyloidosis (P = .017). Diffuse soft tissue uptake with photon defects in the liver area was found only in ATTR amyloidosis.
This study showed that 99mTc-DPD scintigraphy might have capacity to differentiate between AL and ATTR subtypes with good sensitivity in various organs involving primary systemic AL and ATTR amyloidosis. Additional SPECT/CT significantly improved the diagnostic efficacy of 99mTc-DPD scintigraphy.
1. Introduction
Amyloidosis refers to a group of diseases characterized by deposition of proteinaceous fibrils composed of low-molecular weight subunits of a variety of serum proteins (i.e., amyloid), which causes harm and loss of function to affected tissues or organs.[1–3] The disease can be systemic or organ-specific [3–6] and is divided into various subtypes based on the precursor protein, majority of deposits, tissue distribution, and extent of deposition.[1,7–9] Among these, precursor protein is the most importantly used characteristic because it is directly related to treatment and prognosis.[1,10]
Accurate characterization of amyloidosis is essential because the clinical course, process of diagnostic work up, treatment and prognosis vary by subtype.[2–4] For that, tissue biopsy provides precise information regarding subtypes of amyloidosis, there is also a need for a noninvasive imaging modality that can screen the whole body to evaluate disease extent and treatment response. Imaging studies including nuclear medicine have played an important role in amyloidosis, especially in identifying the type of amyloidosis, extent of disease, and response to treatment.[1,2,11]
Several radiotracers for nuclear medicine images have been tested and utilized for amyloidosis.[3]123I-Serum amyloid P component (SAP) scintigraphy has been used for non-invasive diagnosis, monitoring, and treatment response in patients with amyloid light-chain (AL), amyloid A (AA), and cases of transthyretin (ATTR) amyloidosis.[12–16] However, low-quality cardiac visualization, high cost, and limited availability of purified SAP and 123I are major disadvantages to its use.[6,12,17–19] There are several articles for 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT), which has established its usefulness in the field of oncology, while clinical value have not been proven in amyloidosis. Technetium-labeled radiotracers have been regarded as useful tools with advantages of convenient use and lower price, and notable previous studies using technetium-labeled agents such as 99mTc-pyrophosphate (99mTc-PYP), 99mTc-hydroxymethylene diphosphate (99mTc-HDP), and 99mTc-3,3-diphosphono-1,2-pyrophosphate (99mTc-DPD) have distinguishing ability of the subtypes of cardiac amyloidosis.[2,6,10,20–24] However, studies showing disease extent throughout the whole body using scintigraphy are scarce, even though amyloidosis is a systemic disease.
One of the major advantages of nuclear medicine is that it enables the evaluation of the whole body at once. A comprehensive understanding of the precise scintigraphy findings could help identify involved organs, assess disease burden, and select methods for further evaluation. Therefore, we prospectively investigated the imaging findings of 99mTc-DPD scintigraphy in patients with primary systemic AL or ATTR amyloidosis.
2. Methods
2.1. Study subjects
From December 2012 to December 2014, 31 subjects with suspected or alleged amyloidosis prospectively underwent 99mTc-DPD scintigraphy. Among these patients, 10 were excluded because of other pathological subtype (2, AA subtype), no pathological report (3), or previous treatment before the scan (5). Thus, a total of 21 subjects with pathologically confirmed primary systemic AL or ATTR amyloidosis were finally enrolled for analysis, and medical records were reviewed. Our institutional review board approved the study protocol of this prospective study, and informed consent was acquired from all subjects.
2.2. 99mTc-DPD scintigraphy and SPECT/CT
Anterior and posterior whole body scans were performed 3 to 4 hours after intravenous administration of 740 MBq 99mTc-DPD using a dual-headed gamma camera equipped with low-energy, high-resolution collimators at a scan speed of 21 cm/min. If there was suspicious abnormal soft tissue 99mTc-DPD uptake, regional single-photon emission computed tomography/computed tomography (SPECT/CT) was performed for that radioactive lesion using a hybrid SPECT/CT gamma camera (Siemens, SYMBIA T-16). SPECT acquisition settings were matrix size 128 × 128 and time per view of 25 seconds. The equipment had a dual detector with 180 degrees of rotation and step and shoot modes. The data was reconstructed using iterative reconstruction with a Gaussian filter applying scatter correction. Regional CT was performed by a continuous spiral technique after SPECT, using a section width of 0.5 mm, flexible scan time, 130 kV voltage, and matrix size of 128 × 128. No intravenous or oral contrast material was used. Attenuation correction was based on CT-acquired attenuation maps.
2.3. Image analysis
Whole body planar and regional SPECT/CT images were acquired in all subjects. Two nuclear medicine physicians interpreted the planar and regional SPECT/CT images in consensus. For involved organs, 99mTc-DPD uptake was visually and semi-quantitatively evaluated with a 4-point scale (grade 0: no significant uptake or no difference from physiologic uptake, grade 1: uptake less than spine, grade 2: uptake similar to spine, and grade 3: uptake greater than spine). In addition, the presence of diffusely increased soft tissue uptake was evaluated.
2.4. Statistical analysis
Comparison of continuous variables with known normal values in 1 group was performed with a one-sample T-test. Comparison of continuous variables between 2 groups was performed with the Mann-Whitney U test. For comparison between 3 or more groups, the Kruskall–Wallis test was used for continuous variables and Pearson's chi-square tests for nominal variables. A P value ≤ .05 was regarded as significant. Statistical analysis was performed using SPSS Statistics 19.0.0 for Windows (IBM Corporation, Somers, NY).
3. Results
3.1. Clinical and laboratory characteristics
The clinical and laboratory characteristics of our study subjects were summarized in Table 1. A total of 21 subjects (mean age 62.1 ± 11.1 years, and 66.6% males) were all pathologically diagnosed with amyloidosis: AL in 16 and ATTR in 5. The proportion of males was slightly higher, though the difference was not statistically significant. Allowing multiple responses, the most frequent symptom in both subtypes was dyspnea (n = 11), followed by generalized edema (n = 8), discomfort or pain in the lower extremities (n = 5), and foamy urine (n = 2).
Table 1.
Clinical and Laboratory Characteristics of Patients with AL or ATTR Amylodosis.
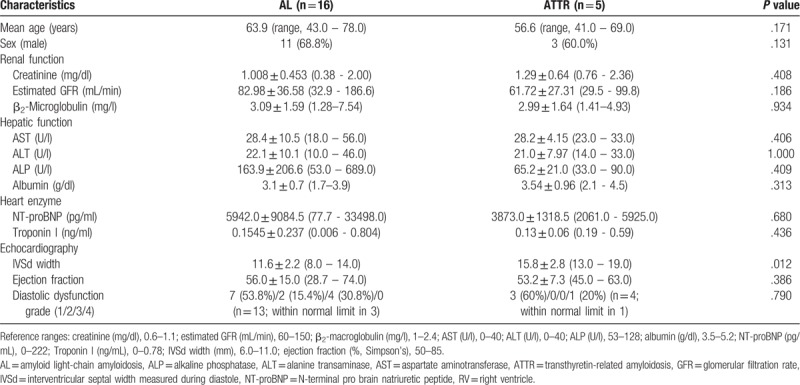
In AL amyloidosis, most patients had nonspecific symptoms including dyspnea, fatigue, general weakness/edema, or foamy urine. Serologic results showed significantly elevated N-terminal pro brain natriuretic peptide (NT-proBNP) level (P = .024) with a variable degree of diastolic cardiac dysfunction. Serum albumin level had lower tendency than group of ATTR, although without statistical significance (P = .056). For echocardiographic parameters, intraventricular septal width measured during diastole was significantly thicker than normal reference (P < .0001). Fourteen patients had been diagnosed with combined multiple myeloma (87.5%) in cases of systemic AL amyloidosis without any other simultaneous malignancy.
In the ATTR group, most patients complained about dyspnea and edema. Serologic results also showed elevated creatinine, β2-macroglobulin, and NT-proBNP, also with a variable degree of diastolic dysfunction. For echocardiographic parameters, intraventricular septal width measured during diastole showed a thicker than normal value.
In comparison of the 2 subtypes, there was no significant difference in age (63.9 ± 10.9 vs 56.6 ± 11.1 y, P = .171), sex (male, 68.8% vs 60.0%, P = .131), or any serologic test. In echocardiographic findings, patients with ATTR subtype had a significantly thicker intraventricular septal width than those with AL subtype (11.6 ± 2.2 vs 15.8 ± 2.8 mm, P = .012) (Table 1).
3.2. 99mTc-DPD scintigraphy and SPECT/CT findings
There were a total of 41 organs from 21 patients diagnosed with amyloidosis pathologically and clinically. All patients had at least 1 involved organ confirmed histopathologically. There were 29 involved organs in patients with AL amyloidosis: 11 hearts, 11 kidneys, 4 livers, and 1 skin. Among the above organs, 18 were histopathologically confirmed. In the remaining 11 organs, involvement was diagnosed by chest/abdominopelvic CT, heart MR, or ultrasonography; this showed involvement of 4 kidneys, 3 hearts, 2 livers, 1 bowel, and 1 lung. There were 12 involved organs in patients with ATTR amyloidosis: five 5 hearts, 4 bowels, 2 kidneys, and 1 lung (Table 2). Among them, 6 organs were histopathologically confirmed. In the remaining 6 organs, involvement was diagnosed by the same imaging modalities as used for AL amyloidosis; 4 bowels, 1 kidney, and 1 lung. Details on each case are showed in Supplementary Table.
Table 2.
Sensitivity of 99mTc-DPD scintigraphy to detect involved organs before treatment in patients with AL or ATTR amyloidosis.
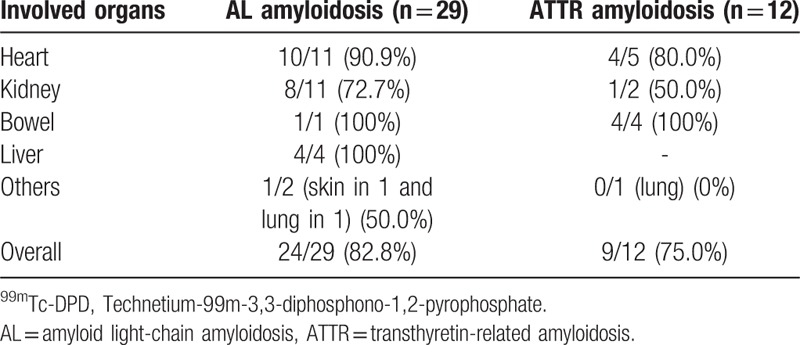
Significant 99mTc-DPD uptake was shown in 33 involved organs of all 41 organs independent subtype (sensitivity = 80.5%); 82.8% in AL and 75.0% in ATTR (Table 2). For the pathologically proven affected organs, significant 99mTc-DPD uptake was found in 19/24 organs (sensitivity = 79.2%); 84.6% in the heart (11/13), 62.5% in the kidney (5/8), 100% in the liver (2/2), and 100% in the skin (1/1). For the clinically diagnosed organs, 14/17 showed significant 99mTc-DPD uptake (sensitivity = 82.4%); 100% in the heart (3/3), 80.0% in the kidney (4/5), 100% in the liver (2/2), 100% in the bowel (5/5), and 0% in the lung (0/2). There was no significant difference in sensitivity between cardiac involvement and that in other organs (87.5%, 14/16 vs 76.0%, 19/25; P = .365).
In AL amyloidosis, significant 99mTc-DPD uptake was found in 15 of the pathologically-proven 18 affected organs (sensitivity = 83.3%); 87.5% in the heart (7/8; grade 1 in 6, grade 2 in 1), 71.4% in the kidney (5/7; grade 1 in 3, grade 2 in 1, and grade 3 in 1), 100% in the liver (2/2; grade 1 in 1 and grade 3 in 1), and 100% in the skin (1/1; grade 1 in 1). Although pathological study was not performed, 9 of 11 additional organs clinically suspected of AL amyloidosis involvement revealed 99mTc-DPD uptake (81.1%; 3/4 in the kidney with grade 1 in 2 and grade 2 in 1, 3/3 in the heart all with grade 3, 2/2 in the liver with grade 1 in 1 and 3 in 1, 1/1 in the bowel with grade 3, and 0/1 in the lung) (Table 3). The sensitivity for detecting cardiac involvement (10/11, 90.9%) was higher than that for detecting other organ involvement (14/18, 77.8%), though without statistical difference (P = .364).
Table 3.
DPD uptake grades of involved organs with AL or ATTR amyloidosis.
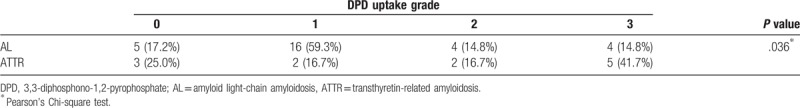
In ATTR, significant 99mTc-DPD uptake was found in 4 of 6 pathologically-proven affected organs (sensitivity = 66.7%); 80.0% in the heart (4/5; grade 3 in 4) and 0% in the kidney (0/1). Although pathological study was not performed, 5 of 6 additional organs clinically suspected of ATTR amyloidosis involvement revealed 99mTc-DPD uptake (83.3%, 4/4 in the bowel with grade 1 in 1, grade 2 in 2, and grade 3 in 1; 1/1 in the kidney with grade 1; and 0/1 in the lung) (Table 3). The uptake grade of involved heart was higher than that of other involved organs with borderline statistical significance (3.0 ± 0 vs 1.8 ± 0.8; P = .113). There was no significant difference in sensitivity between involved organs (80.0% and 4/5 for the heart vs 71.4%, 5/7 for other organs; P = .735). In addition, diffusely increased soft tissue uptake, except in the liver area, was found in all patients with ATTR subtype, but not in those with AL subtype (Figs. 1–3).
Figure 1.
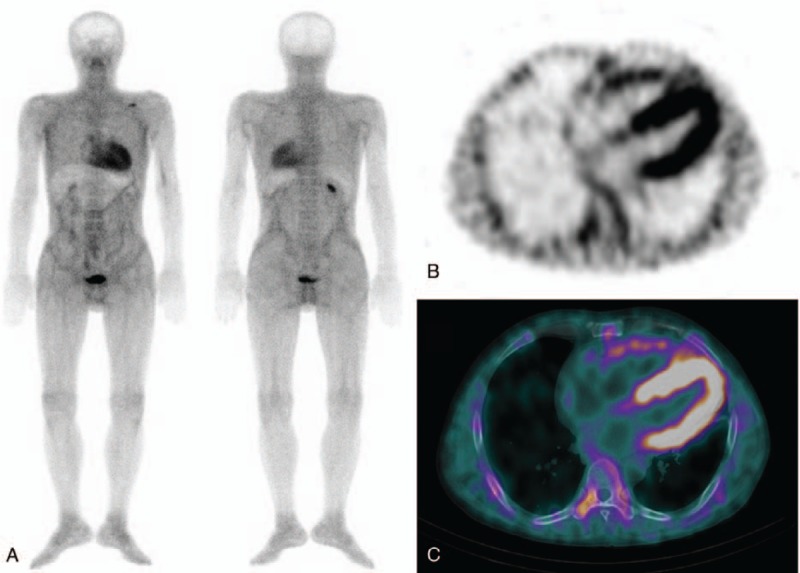
(A) Anterior and posterior whole body 99mTc-DPD scan images of a 50-year-old male patient with ATTR subtype of cardiac amyloidosis show diffusely increased soft tissue and cardiac uptake with a photon defect in the liver. Skeletal uptake is relatively decreased compared to the usual bone scans. (B and C) Additional SPECT/CT image is helpful to validate that cardiac uptake corresponded to the myocardium, suggesting amyloidosis.
Figure 3.
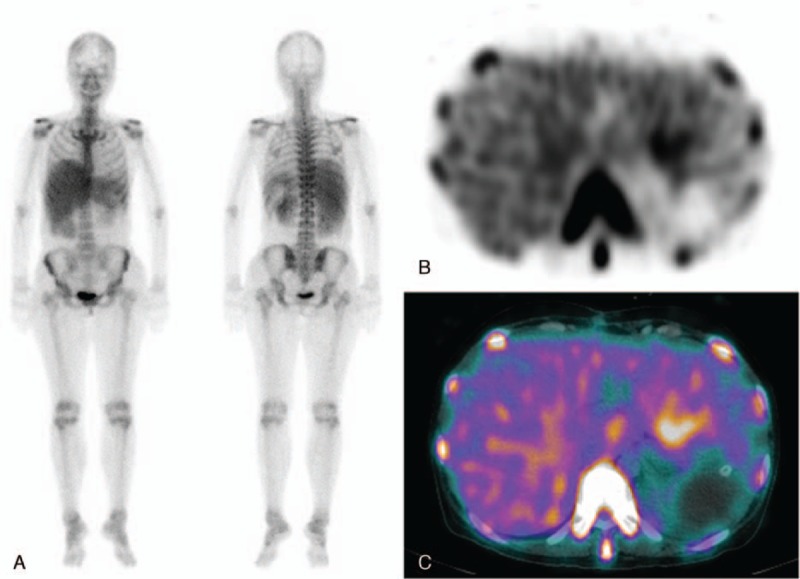
(A) Anterior and posterior whole body 99mTc-DPD scan images of a 42-year-old female patient with AL subtype amyloidosis involving the liver and kidneys show diffusely increased 99mTc-DPD uptake in the liver and both kidneys. However, it is not clear whether there is increased splenic uptake because of hepatomegaly. (B and C) Additional SPECT/CT image is helpful to clarify that the 99mTc-DPD uptake is confined to the enlarged liver.
In comparing the 2 subtypes in 99mTc-DPD uptake grading, grade 2 (59.3%) had the highest percentage of AL subtypes for involved organs. On the other hand, grade 4 (41.7%) had a greater percentage of ATTR subtype (P = .036, Table 3). In other words, the uptake grade of the ATTR subtype was significantly higher than that of the AL subtype (1.24 ± 0.91 vs 1.75 ± 1.28; P = .017).
In 20 of the 41 involved organs (48.8%; 9 hearts, 6 kidneys, 4 livers, and 1 bowel), additional SPECT/CT images were helpful to determine whether there was abnormal soft tissue 99mTc-DPD uptake related to amyloidosis on planar imaging. 18 organs (9 hearts, 6 kidneys, 2 livers, and 1 bowel) with equivocal or no definite DPD uptake in planar imaging, eventually were confirmed with mild DPD uptake by SPECT/CT (Fig. 3). In the remaining 2 organs, SPECT/CT was also helpful to ensure abnormal DPD uptake in involved organs, which were uncertain by attenuation in planar imaging (Fig. 2).
Figure 2.
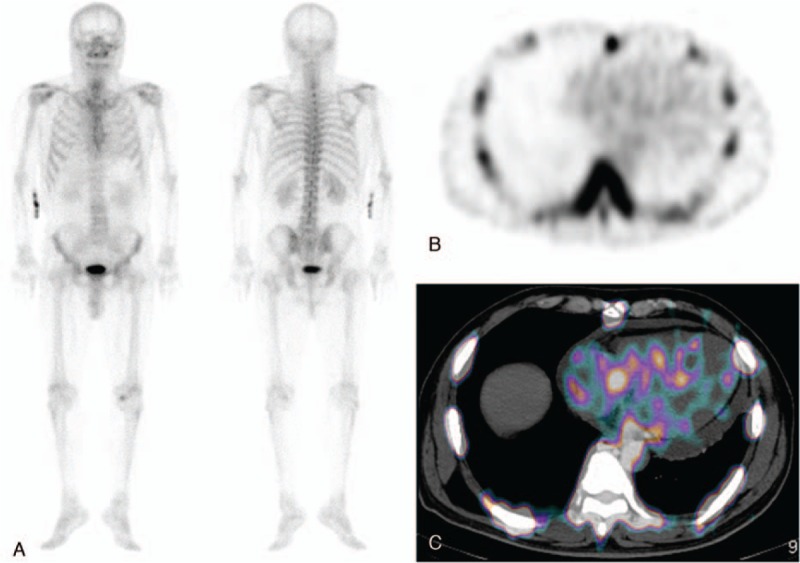
(A) Anterior and posterior whole body 99mTc-DPD scan images of a 64-year-old male patient with AL subtype of cardiac amyloidosis show equivocal mild 99mTc-DPD uptake in the cardiac area. It is unclear whether that uptake is related to cardiac amyloidosis because there was pericardial effusion on chest X-ray and echocardiography. (B and C) On the additional SPECT/CT image, the 99mTc-DPD uptake does correspond to the myocardium, suggesting cardiac amyloidosis. On the contrary, there is no significant 99mTc-DPD uptake in the pericardial effusion on the SPECT/CT image.
4. Discussion
Amyloidosis is a systemic disease with various subtypes that have different treatments and prognoses. Tissue confirmation is essential for diagnosis but is not only imperfect for overall evaluation of this systemic disease, but also limited for patients who are intolerable to invasive procedures. Since amyloidosis is a systemic disease, and organ-related symptoms appear only after affected organ dysfunction has started, identifying involved organs in subclinical status is important for timely proper treatment before organ failure. However, because there are poor choices of imaging modalities for suspicious organs, even after pathologic confirmation, sometimes an additional biopsy of suspected involved organs is required to confirm amyloidosis involvement. In the present study, whole body 99mTc-DPD scintigraphy with additional SPECT/CT for patients with pathologically-proven AL or ATTR amyloidosis showed good sensitivity for identifying involved organs and helped to characterize the subtype of amyloidosis. Especially, additional SPECT/CT images were helpful to determine the presence of abnormal 99mTc-DPD uptake suggesting amyloidosis and improved the sensitivity for detecting the involved organs over planar images.
Because the major cause of death in amyloidosis is related to heart involvement, early and accurate diagnosis of cardiac amyloidosis followed by appropriate therapy is clinically important.[25–28] Although echocardiography or magnetic resonance imaging are choice of image modalities for cardiac amyloidosis, precise diagnosis of cardiac involvement and differentiating subtypes with non-invasive diagnostic tools remain difficult, with limited ability in the early phase.[2,6,29–31] In the present study, 99mTc-DPD scintigraphy with regional SPECT/CT showed a high sensitivity of 87.5% of cardiac involvement in both of AL and ATTR patients with preserved left ventricular ejection fraction and normal or mild diastolic dysfunction. Our results were different from those of previous studies showing no significant 99mTc-DPD uptake in hearts with AL amyloidosis, even by regional SPECT.[6,19,32] This difference may be explained by the use of regional SPECT/CT in our study; the first is precise attenuation correction of heart, which is deep anatomic location of the chest, and second is accurate anatomic correlation to myocardial uptake, especially in cases of combined pericardial effusion. These advantages may contribute to the relative higher sensitivity of our study.
Except in cardiac amyloidosis, 99mTc-DPD uptake in extra-cardiac amyloid deposits is not fully described. In the present study, extra-cardiac 99mTc-DPD uptake was observed in various organs with clinically suspected amyloidosis including the kidney, bowel, liver, and skin, showing moderately high sensitivity in both AL and ATTR amyloidosis (77.8% for AL and 71.4% for ATTR). In particular, there was high uptake in hepatic AL amyloidosis and bowel amyloidosis in both AL and ATTR types. Like cardiac amyloidosis, SPECT/CT was helpful to identify abnormal 99mTc-DPD uptake based on accurate anatomic localization and attenuation correction.
Extraosseous accumulation of 99mTc-phosphate derivatives, including 99mTc-DPD, is related to expanded interstitial volume, hyperemia, and a high local concentration of metals such as iron or calcium. However, the precise uptake mechanism for amyloidosis is still unclear. Since amyloidosis is characterized by abnormal deposition of amyloid in the interstitium of affected tissues or organs, it may cause increased interstitial volume and passive localization of 99mTc-phosphate derivatives as a consequence of dynamic equilibrium with blood.[33] Also, because the kidney is the most commonly affected organ and the major excretory route of 99mTc-DPD, blood concentration of 99mTc-phosphate derivatives might remain high in patients with renal impairment. This is another strong hypothesis for the well-visualized extraosseous uptake of involved organs in patients with amyloidosis. Moral et al. have suggested that cardiac uptake of 99mTc-DPD in AL amyloidosis could be related to radiotracer in the bloodstream secondary to reduced clearance by heart failure rather than true specific deposition in the myocardium.[6] Another possible mechanism is dystrophic calcification that could occur in degenerated or necrotic tissue, resulting in breakage of cell membranes, allowing influx of calcium into cells and accumulation of 99mTc-phosphate derivatives in the affected organs.[33] This possible mechanism was also supported by the normal serum calcium levels in all patients in our study (serum calcium = 8.6 ± 1.0 mg/dl, reference range = 8.4–10.2 mg/dl). However, these are general hypotheses for the extraosseous accumulation of 99mTc-phosphate derivatives, which may not explain the different 99mTc-DPD uptake patterns between AL and ATTR amyloidosis.
There was a higher cardiac uptake in ATTR than AL amyloidosis in the present study, and these findings are consistent with previous studies.[2,19] An additional noteworthy feature of 99mTc-DPD scintigraphy in our study was higher soft tissue uptake in only ATTR amyloidosis. In all 5 cases with ATTR subtype, 99mTc-DPD scans showed not only strong cardiac uptake, but also diffusely increased soft tissue uptake with a photon defect in the liver. These findings were not seen in AL amyloidosis. These findings may be used as key diagnostic criteria for differentiating between ATTR and AL, along with cardiac uptake grade in 99mTc-DPD scintigraphy. Previous studies have shown that the TTR mutation has high affinity for serum HDL, and this affinity causes conglomeration and deposition of the TTR protein in tissues organs.[34] Andersson et al have shown that these lipophilic depositions of TTR molecules can passively bind to the cell membrane, may have oxidative toxic effects by interfering with cytoplasmic signaling pathways, and induce cell apoptosis in a neuroblastoma cell line.[35] Further, this deposition could cause high concentrations of calcium in affected sites due to damaged cells and transchelation of 99mTc-phosphate derivatives.[33] Also, abnormal deposition of TTR molecules could activate scavenger systems and cause secondary hyperemia. Perugini et al. have suggested another hypothesis that 99mTc-DPD has a direct affinity for specific fragments of TTR protein, since there was strong 99mTc-DPD uptake in only ATTR cardiac amyloidosis,[19] although this has not been demonstrated at the cellular level. We consider that multiple mechanisms might be involved in the higher 99mTc-DPD uptake in ATTR.
This study has several limitations. First, this was a single institutional study with a relatively small number of subjects. Second, pathologic confirmation was not performed in all clinically involved organs, which might produce bias regarding the sensitivity of 99mTc-DPD scanning. Further study with a larger subject pool is warranted.
5. Conclusion
The results of this study confirmed that significant 99mTc-DPD uptake was observed in various organs with primary systemic AL or ATTR amyloidosis with good sensitivity in whole body scintigraphy with additional SPECT/CT. Additional SPECT/CT significantly improved the diagnostic efficacy of 99mTc-DPD scintigraphy. Uptake grade of involved organs and degree of background activity might help to differentiate between AL and ATTR subtypes.
Author contributions
Conceptualization: Joohee Lee, Kihyun Kim, Seok Jin Kim.
Data curation: Joohee Lee, Jin-Oh Choi, Joon Young Choi.
Formal analysis: Joohee Lee, Kihyun Kim, Jin-Oh Choi, Seok Jin Kim, Joon Young Choi.
Funding acquisition: Kihyun Kim, Seok Jin Kim, Joon Young Choi.
Investigation: Joohee Lee, Kihyun Kim, Jin-Oh Choi, Seok Jin Kim, Eun-Seok Jeon, Joon Young Choi.
Methodology: Joohee Lee, Kihyun Kim, Seok Jin Kim, Joon Young Choi.
Software: Joon Young Choi.
Supervision: Kihyun Kim, Seok Jin Kim, Eun-Seok Jeon, Joon Young Choi.
Validation: Kihyun Kim.
Visualization: Jin-Oh Choi.
Writing – original draft: Joohee Lee, Joon Young Choi.
Writing – review & editing: Joohee Lee, Kihyun Kim, Jin-Oh Choi, Seok Jin Kim, Eun-Seok Jeon, Joon Young Choi.
Supplementary Material
Footnotes
Abbreviations: 99mTc-DPD = 99mTc-3,3-diphosphono-1,2-pyrophosphate, AA = amyloid A, AL = amyloid light-chain, ATTR = transthyretin, SPECT/CT = single-photon emission computed tomography/computed tomography.
How to cite this article: Lee J, Kim K, Choi JO, Kim SJ, Jeon ES, Choi JY. 99mTc-DPD scintigraphy and SPECT/CT in patients with AL and ATTR Type Amyloidosis: Potential clinical implications. Medicine. 2020;99:4(e18905).
JL and KK contributed equally to this work.
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120175).
The authors declare that they have no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Glaudemans AW, Slart RH, Noordzij W, et al. Utility of 18F-FDG PET/CT in patients with systemic and localized amyloidosis. Eur J Nucl Med Mol Imaging 2013;40:1095–101. [DOI] [PubMed] [Google Scholar]
- [2].Glaudemans AW, van Rheenen RW, van den Berg MP, et al. Bone scintigraphy with 99mtechnetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid 2014;21:35–44. [DOI] [PubMed] [Google Scholar]
- [3].Aljaroudi WA, Desai MY, Tang WH, et al. Role of imaging in the diagnosis and management of patients with cardiac amyloidosis: State of the art review and focus on emerging nuclear techniques. J Nucl Cardiol 2014;21:271–83. [DOI] [PubMed] [Google Scholar]
- [4].Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci 2001;356:203–10. discussion 10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Glaudemans AW, Slart RH, Zeebregts CJ, et al. Nuclear imaging in cardiac amyloidosis. Eur J Nucl Med Mol Imaging 2009;36:702–14. [DOI] [PubMed] [Google Scholar]
- [6].de Haro-del Moral FJ, Sanchez-Lajusticia A, Gomez-Bueno M, et al. Role of cardiac scintigraphy with 99mTc-DPD in the differentiation of cardiac amyloidosis subtype. Rev Esp Cardiol (Engl Ed) 2012;65:440–6. [DOI] [PubMed] [Google Scholar]
- [7].Pepys MB. Amyloidosis. Annu Rev Med 2006;57:223–41. [DOI] [PubMed] [Google Scholar]
- [8].Westermark P. Localized AL amyloidosis: a suicidal neoplasm? Ups J Med Sci 2012;117:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baqir M, Lowe V, Yi ES, et al. 18F-FDG PET scanning in pulmonary amyloidosis. J Nucl Med 2014;55:565–8. [DOI] [PubMed] [Google Scholar]
- [10].Bokhari S, Castano A, Pozniakoff T, et al. 99mTc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013;6:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hutt D, McphillipscH, Mcknight S, et al. DPD Scintigraphy for diagnosis of amyloidosis in 1191 patients– a single centre experience. Orphanet J Rare Dis 2015;10: Suppl 1: O16. [Google Scholar]
- [12].Hazenberg BP, van Rijswijk MH, Lub-de Hooge MN, et al. Diagnostic performance and prognostic value of extravascular retention of 123I-labeled serum amyloid P component in systemic amyloidosis. J Nucl Med 2007;48:865–72. [DOI] [PubMed] [Google Scholar]
- [13].Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med 1990;323:508–13. [DOI] [PubMed] [Google Scholar]
- [14].Hachulla E, Maulin L, Deveaux M, et al. Prospective and serial study of primary amyloidosis with serum amyloid P component scintigraphy: from diagnosis to prognosis. Am J Med 1996;101:77–87. [DOI] [PubMed] [Google Scholar]
- [15].Hazenberg BP, van Rijswijk MH, Piers DA, et al. Diagnostic performance of 123I-labeled serum amyloid P component scintigraphy in patients with amyloidosis. Am J Med 2006;119: 355 e15-24. [DOI] [PubMed] [Google Scholar]
- [16].Hawkins PN. Serum amyloid P component scintigraphy for diagnosis and monitoring amyloidosis. Curr Opin Nephrol Hypertens 2002;11:649–55. [DOI] [PubMed] [Google Scholar]
- [17].Mekinian A, Jaccard A, Soussan M, et al. 18F-FDG PET/CT in patients with amyloid light-chain amyloidosis: case-series and literature review. Amyloid 2012;19:94–8. [DOI] [PubMed] [Google Scholar]
- [18].Park CH, Kim HS, Shin HY, et al. Hepatic uptake of Tc-99m MDP on bone scintigraphy from intravenous iron therapy (Blutal). Clin Nucl Med 1997;22:762–4. [DOI] [PubMed] [Google Scholar]
- [19].Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005;46:1076–84. [DOI] [PubMed] [Google Scholar]
- [20].Falk RH, Lee VW, Rubinow A, et al. Sensitivity of technetium-99m-pyrophosphate scintigraphy in diagnosing cardiac amyloidosis. Am J Cardiol 1983;51:826–30. [DOI] [PubMed] [Google Scholar]
- [21].Falk RH, Lee VW, Rubinow A, et al. Cardiac technetium-99m pyrophosphate scintigraphy in familial amyloidosis. Am J Cardiol 1984;54:1150–1. [DOI] [PubMed] [Google Scholar]
- [22].Wizenberg TA, Muz J, Sohn YH, et al. Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the noninvasive diagnosis of cardiac amyloidosis. Am Heart J 1982;103:468–73. [DOI] [PubMed] [Google Scholar]
- [23].Ak I, Vardareli E, Erdinc O, et al. Myocardial Tc-99m MDP uptake on a bone scan in senile systemic amyloidosis with cardiac involvement. Clin Nucl Med 2000;25:826–7. [DOI] [PubMed] [Google Scholar]
- [24].Kulhanek J, Movahed A. Uptake of technetium-99m HDP in cardiac amyloidosis. Int J Cardiovasc Imaging 2003;19:225–7. [DOI] [PubMed] [Google Scholar]
- [25].Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid 2006;13:236–49. [DOI] [PubMed] [Google Scholar]
- [26].Obici L, Merlini G. An overview of drugs currently under investigation for the treatment of transthyretin-related hereditary amyloidosis. Expert Opin Investig Drugs 2014;23:1239–51. [DOI] [PubMed] [Google Scholar]
- [27].Benson MD, Pandey S, Witchell D, et al. Antisense oligonucleotide therapy for TTR amyloidosis. Amyloid 2011;18: Suppl 1: 60. [DOI] [PubMed] [Google Scholar]
- [28].Leung N, Nasr SH, Sethi S. How I treat amyloidosis: the importance of accurate diagnosis and amyloid typing. Blood 2012;120:3206–13. [DOI] [PubMed] [Google Scholar]
- [29].Rapezzi C, Quarta CC, Guidalotti PL, et al. Role of 99mTc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 2011;4:659–70. [DOI] [PubMed] [Google Scholar]
- [30].Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med 1997;337:898–909. [DOI] [PubMed] [Google Scholar]
- [31].Trikas A, Rallidis L, Hawkins P, et al. Comparison of usefulness between exercise capacity and echocardiographic indexes of left ventricular function in cardiac amyloidosis. Am J Cardiol 1999;84:1049–54. [DOI] [PubMed] [Google Scholar]
- [32].Banypersad SM, Moon JC, Whelan C, et al. Updates in cardiac amyloidosis: a review. J Am Heart Assoc 2012;1:e000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Worsley DF, Lentle BC. Uptake of technetium-99m MDP in primary amyloidosis with a review of the mechanisms of soft tissue localization of bone seeking radiopharmaceuticals. J Nucl Med 1993;34:1612–5. [PubMed] [Google Scholar]
- [34].Liu JP, Wang QY, Zheng F, et al. Effect of MPO/H2O2/NO(-) system on nitric oxide-mediated modification of TTR amyloid and serum TTR in FAP ATTR Val30Met patients. Genet Mol Res 2014;13:2368–76. [DOI] [PubMed] [Google Scholar]
- [35].Andersson K, Olofsson A, Nielsen EH, et al. Only amyloidogenic intermediates of transthyretin induce apoptosis. Biochem Biophys Res Commun 2002;294:309–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


