Background.
Graft survival rates after intestinal transplantation (ITx) are still the lowest in comparison to other solid organ transplants. One of the main reasons is the frequent occurrence of acute cellular rejection (ACR). Vedolizumab is an antibody against α4β7+ integrin involved in gut-homing of T cells which has been approved for inflammatory bowel diseases (IBD). We report its off-label use to treat ACR after ITx.
Methods.
Following abdominal wall transplantation (AWTx) and ITx, clinical course was followed biochemically. Sequential small intestinal biopsies were taken preceding, during, and after ACR treatment with vedolizumab, following the standard therapy regime for IBD. Rejection was diagnosed histologically, and proinflammatory (α4β7+, interleukin-17+) and regulatory (FoxP3+) T cells were analyzed by immunohistochemistry.
Results.
ACR in both the ITx and AWTx resolved upon vedolizumab treatment, which was safe, evidenced by clearing an astrovirus and primary cytomegalovirus infection. Only a slight reduction of α4β7+ cells in the mucosa was observed, and α4β7+ and regulatory T cells could still move into the lamina propria upon infection.
Conclusions.
Vedolizumab is a safe treatment option for ACR after ITx but its mechanism is probably not only based on inhibition of gut-selective T-cell homing.
Intestinal transplantations (ITx) have already been performed years, but its graft survival rates after 5 years have plateaued at approximately 50% in the past decade.1 One of the main causes of graft loss is acute cellular rejection (ACR),2 characterized by gut homing of inflammatory cells after priming with donor-derived antigens.3,4 This results in a mixed inflammatory infiltrate in the lamina propria consisting mostly of mononuclear cells accompanied by apoptosis of crypt epithelial cells and epithelial cell damage.5
Gut homing of inflammatory cells is one of the main features in ITx that also occurs in other intestinal diseases, such as intestinal graft-versus-host disease and inflammatory bowel disease (IBD).6,7 It requires a set of signaling molecules that are responsible for trafficking of leukocytes specifically to the intestine, including α4β7 integrin.8 This integrin is highly expressed by proinflammatory gut T- and B cells and eosinophils.8 Its ligand, mucosal addressin cell adhesion molecule-1, is overexpressed in endothelial cells of venules in the gut’s lymphoid organs and mucosa during inflammation.9 Vedolizumab, a humanized mouse anti-α4β7 monoclonal antibody (Entyvio; Takeda Pharmaceutical Company, Tokyo, Japan) shows therapeutic efficacy in IBD,10 as well as in other immune-mediated intestinal diseases, such as collagenous colitis and eosinophilic gastroenteritis.11–14 It is believed to be gut specific because of its exclusive interaction with the heterodimer of the aforementioned integrin, thereby blocking the influx of inflammatory cells into the gut.15 More recent data suggest that vedolizumab might not necessarily work on the acquired immune system but also on the innate system.16
Current treatment of ACR is focused on suppressing systemic T-cell proliferation and/or depletion, but often this is not successful and the rejecting graft needs to be removed.17 Thus, alternative pharmacological approaches are urgently needed to treat ACR after ITx and, considering its mechanism of action, vedolizumab could be a promising option.
Here, we describe the intraintestinal cellular dynamics of a combined ITx and abdominal wall transplantation (AWTx) patient with ACR of both grafts who did not respond to regular immunosuppressive therapy and was subsequently safely and successfully treated with vedolizumab.
MATERIALS AND METHODS
Ethical Approval
Treatment and follow-up studies were fully understood and accepted by the patient and approved by the Ethical Committee of the University Medical Center Groningen (study number M14.163082).
Immunohistochemistry
Hematoxylin and eosin slides were prepared according to a standard protocol to diagnose graft rejection. Paraffin-embedded tissue sections of the intestinal biopsies gathered by endoscopy were cut (4 µm) from routine diagnostic blocks, placed on Starfrost slides (3054-1, Klinipath, VWR, Breda, The Netherlands), dried, deparaffinized in xylene, and rehydrated in alcohol. Endogenous peroxidase was blocked with 0.3% H2O2 in phosphate-buffered solution (PBS) for 30 minutes. The slides were then blocked for 30 minutes with 1% bovine serum albumin (BSA)/PBS before being incubated for 1 hour at room temperature with a primary antibody against FoxP3 (1:100 Abcam [22510], Cambridge, UK) and interleukin (IL)-17 (1:200 R&D Systems [AF-317-NA], Minneapolis, MN, USA). The secondary and tertiary steps were performed with horseradish peroxidase-labeled antibodies (1:50, Dako, Agilent, Santa Clara, CA, USA; rabbit antimouse and goat antirabbit, respectively) in 1% BSA/PBS supplemented with 1% human serum, incubated for 30 minutes. Binding was detected by 3,3-diaminobenzidine and counterstained with hematoxylin.
The Act-1 (anti-α4β7) antibody was used (1:50 Takeda Pharma A/S, Taastrup, Denmark) for staining of vedolizumab-targeted cells. Frozen intestinal tissue embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek Europe, Alphen aan den Rijn, the Netherlands) was cut, dried, and fixed with 4% paraformaldehyde for 10 minutes. Endogenous peroxidase was blocked with 0.075% H2O2 in PBS for 30 minutes, followed by 30 minutes blocking in 1% PBS/BSA, 1-hour incubation with the primary antibody, followed by the secondary (rabbit antimouse peroxidase-labeled) and tertiary (goat antirabbit peroxidase-labeled) antibodies (1:50, Dako, Agilent) for 30 minutes each. Binding was detected by 3-amino-9-ethylcarbazole and counterstained with hematoxylin.
Definitions
ACR is defined as the presence of ≥6 apoptotic bodies per 10 consecutive crypts (ABC) accompanied by crypt epithelial cell destruction and the presence of inflammatory cells in the lamina propria.5 Areas where there are higher numbers of cells of interest and ABC selected at lower magnification are herewith referred to as hotspots. All biopsies with different types of staining were scanned first for hotspots. If none were found, a random area was chosen (at least 5 per slide). A high-power field is defined at ×40 magnification, with an area of 0.24 mm2.
Cell Counting
Rejection was identified for diagnostic purposes and reported here in that manner, according to the guidelines for each organ.5,18 Cell counting was performed independently by a researcher and a pathologist, and a consensus was reached when discrepancies emerged. All stained cells were counted individually per high-power field. The presence of cytoplasmic staining using the antibodies directed against α4β7, IL-17, and FoxP3 was considered positive for the antibody. The average of all available fields was taken for analyses. Primary data were analyzed using Microsoft Excel (Microsoft, Redmond, Washington, USA), GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA), and SPSS 25 (IBM, Armonk, NY, USA). Measurements were then grouped into clinically relevant periods and presented as the median and range up to 1 year post transplant.
CASE DESCRIPTION AND RESULTS
A 19-year-old female underwent a subtotal colectomy because of ulcerative colitis. Her diagnosis was changed to Crohn’s disease after she presented ulcerations in her oesophagus, stomach, and small bowel, complicated by severe perforations. This led to small bowel resection and consequently an ultrashort bowel syndrome with loss of abdominal domain. She had a distal duodenogastrostomy, drained by a percutaneous gastrostomy.19 After 10 months on home total parenteral nutrition, the patient was screened for ITx because of liver function impairment and jaundice. During the following 2 years, she suffered from several episodes of line infections, malnourishment, and poor quality of life and was put on the waiting list for a combined ITx with AWTx.
IT and AWTx
In March 2015, the patient successfully underwent ITx in combination with full-thickness AWTx (the surgical description has been published elsewhere20). Crossmatch was negative. A list of clinically relevant episodes, immunosuppression, and trough levels is given in Table 1.
TABLE 1.
Clinically relevant episodes and immunomodulatory treatment within the first year post transplant
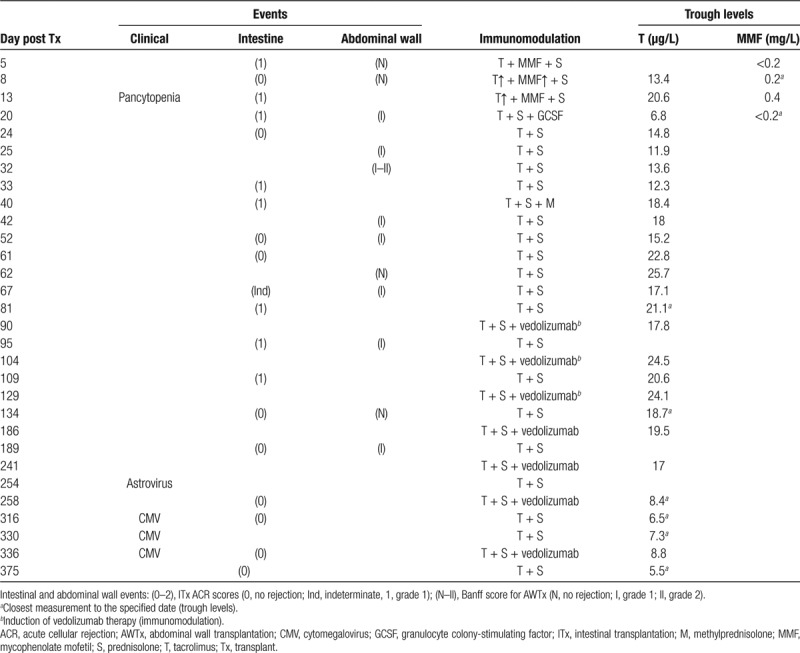
The induction of immunosuppression consisted of methylprednisolone (500 mg) and antithymocyte globulin (ATG; 9 mg/kg). The maintenance plan initially followed a standard scheme consisting of tacrolimus (8 mg/d, trough levels 13–17 mg/L), mycophenolate mofetil (MMF; 2 g/d, trough levels 2–4 mg/L), and prednisolone. The latter drug is administered in the following manner: 2 mg/kg/d intravenous (IV) for days 1–3 post ITx; 1 mg/kg/d IV or oral for days 4–8; 0.3 mg/kg/d oral for days 9–30; 0.2 mg/kg/d oral for months 2–3; and 0.1 mg/kg/d oral for months 4–6. Standard treatment of ACR is performed in the department in a stepwise manner: first by increasing tacrolimus dosage; second by giving a 3-day boost of IV methylprednisolone; third by adding a T-cell depleting agent such as ATG.
On day 6 post transplantation, the ileum biopsy revealed signs of grade 1 ACR, treated temporarily with an increased dose of tacrolimus to reach trough levels between 15–20 µg/L. On day 14, despite adequate trough levels of tacrolimus (20.6 µg/L), ACR returned together with fever and pancytopenia, the latter requiring cessation of MMF treatment. Granulocyte colony-stimulating factor was administered on day 20 (30 million units), resulting in an increase in blood leukocyte count. The transplanted abdominal wall showed no signs of rejection until day 21 (grade 1). ACR persisted and treatment with methylprednisolone (3 d 1000 mg IV) suppressed this for 11 days. ACR (grade 2) returned on day 81. Having considered the previous development of pancytopenia under MMF treatment and limited alternative options, we decided to use vedolizumab because of her history of IBD and its safe and potentially promising mechanism of action.
We treated the patient with 300-mg vedolizumab on weeks 0, 2, and 6 (induction), and every 8 weeks thereafter (maintenance, 8 infusions during the period of this study), with biopsy controls. Immunosuppression with tacrolimus continued alongside this treatment with trough levels between 17.8–24.5 µg/L (normal–high) during induction and 6.5–19.5 µg/L (normal) during maintenance.
Immunosuppression with Vedolizumab
Signs of rejection in the ITx and AWTx grafts disappeared during the induction period, and her clinical status steadily improved during the period of this study (1-y follow-up). This was accompanied by a slight reduction of vedolizumab-targeted cells in the intestinal graft and an increase in IL-17+ Th17 cells (Figure 1). Treatment was safe, since the patient could clear an astrovirus infection on day 259 post ITx, diagnosed by RNA analysis from fecal samples. Remarkably, also a primary cytomegalovirus (CMV) infection (between d 316 and 337 post ITx, IgM and DNA positive in polymerase chain reaction) during valganciclovir prophylaxis cleared without any clinical symptoms. This was accompanied by an increase of α4β7+ (proinflammatory) and FoxP3+ (regulatory T cells, Treg) cells in the graft as well (Figure 1). Maintenance therapy continued with tacrolimus (trough levels 5–7 µg/L) and prednisone (10 mg/d). Both cleared infectious episodes occurred during vedolizumab treatment (between the sixth and seventh infusions of vedolizumab).
FIGURE 1.
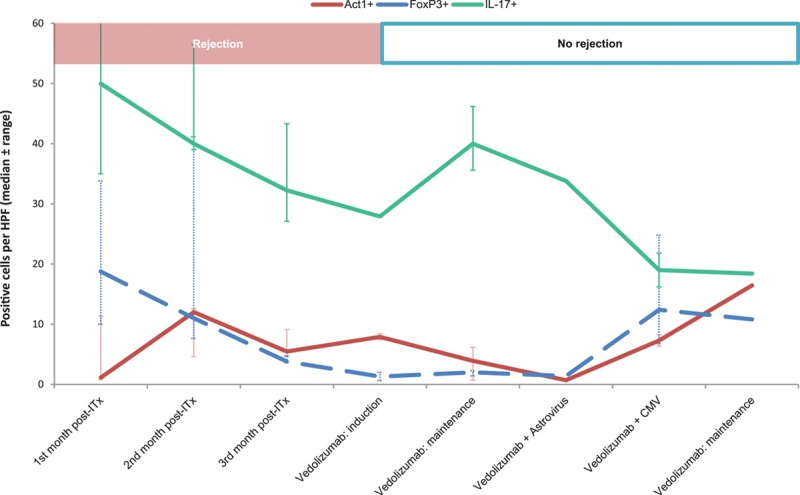
Vedolizumab is safe to use in intestinal transplant recipients to treat ACR. Timeline showing the presence of proinflammatory cells (Act-1+, IL-17+) and Treg (FoxP3+) in the intestinal graft in relationship with the most clinically relevant events during the first year post transplantation. Results are herewith presented in periods as the median and range of positive cells per HPF (see Materials and Methods section for more information). The prevedolizumab era is represented per month, and the vedolizumab treatment period consists of induction period (3 infusions within 2 mo), maintenance without comorbidities (4 mo), primary astrovirus (1 biopsy), and CMV infections (20 d), and the last biopsy before the end of the first year. More details within each period can be found in Table 1. ACR, acute cellular rejection; CMV, cytomegalovirus infection; HPF, high-power field; IL-17, interleukin-17; ITx, intestinal transplantation.
DISCUSSION
This report presents the first patient with ACR after a combined ITx and AWTx who was safely and successfully treated with vedolizumab. Astrovirus and primary CMV infections were uneventfully cleared, and there were no episodes of ACR after the therapy started. This case allowed us to study immune cell dynamics surrounding episodes of infection and rejection of an intestinal and abdominal wall graft treated with an integrin-specific antibody.
This patient had a background of Crohn’s disease, but there were no signs of recurrent disease post-transplantation. Rejection could not be controlled by the standard treatment options with tacrolimus, MMF, or methylprednisolone. Considering her past experience with infliximab and the proposed mechanism of action of vedolizumab, it was chosen as the most promising treatment option. ACR disappeared during induction period alongside a decrease of the drug’s target cells, which then reappeared under maintenance. This suggests that the therapeutic effect of this drug is not solely based on blocking the entry of α4β7+ integrin cells in the intestinal mucosa, as was proposed by others.16,21
The aforementioned dynamics of α4β7+ cells in the graft may indicate that autoreactive leukocytes with increased α4β7+ on their surface that initiated the rejection episode were blocked or downregulated during induction.22 Studies in a large IBD population have shown that there are increased levels of expression of proinflammatory markers such as that of α4 subunit and regulatory molecules for Th17 cells.23 Patients with IBD also have impaired functions of Th17 helper cells, which maintain homeostasis between the intestinal mucosa and the microbiota.16,23 Interestingly, our study showed an increase in the presence of this cell type during induction therapy with vedolizumab, alongside the improvement of her clinical picture. On the other hand, during the infection periods there are α4β7+ cells migrating to the gut and in the maintenance phase an influx of α4β7+ was observed that was not accompanied with rejection. An explanation could be that α4β7 upregulation on leukocytes is less prominent in these situations and that these α4β7+ cells use alternative routes for migration into the gut. Furthermore, in IBD patients, it has been shown that vedolizumab also changes the transcriptional signatures of the innate immune system.16 Additionally, a Crohn’s disease patient with a grade 3 ACR episode and an infliximab-resistant, refractory rejection was also given vedolizumab in another center. This patient’s inflammatory signs (ACR, inflammatory stenosis) also resolved after induction therapy, and in this patient maintenance therapy was not needed (Dr A. Pascher, 2015, written personal communication).
Other cases of ITx ACR treated with vedolizumab were presented at the XV Congress of the Intestinal Rehabilitation and Transplantation Association (CIRTA).24,25 Four pediatric patients with a background of microvillus inclusion disease were transplanted and suffered from ACR. Induction with vedolizumab was successful, but maintenance treatment was not effective (Norsa et al, poster presentation, CIRTA, 2017).25 The underlying pathophysiology of graft rejection may differ in patients with such disease, as they do not suffer from an impaired immune response that is typical for IBD patients.
Our patient suffered from viral infections during this treatment, which resolved without complications, indicating that this drug is safe. Other recent evidence supports this notion.26 CMV primarily affects the endothelial cells of the intestine.27 The union between the integrin α4β7 and the endothelial cell-expressed receptor mucosal addressin cell adhesion molecule-1 is blocked with vedolizumab. The asymptomatic resolution of this primary CMV infection could therefore be associated with a milder, yet effective inflammatory response in the vessel walls.16
One could speculate that the influx of protective Treg should be compromised by this treatment. However, FoxP3+ Treg were still present in the graft during vedolizumab treatment and infiltrated the graft during infections (Figure 1). This may support other studies that show α4β7 levels are relatively low in a specific subset of FoxP3+ Treg,22 and thus may depend on different gut-homing mechanisms.16
Notably, the transplanted abdominal wall of this patient was not a sentinel marker for rejection of the intestinal graft, as observed by others.28 Rejection of the skin started 2 weeks after it was detected in the intestine and resolved after 3 infusions of vedolizumab, later than the intestinal graft. Although vedolizumab is proposed to be gut selective, we also observed the resolution of rejection on the transplanted abdominal wall. Transplanted skin has an inflammatory microenvironment that might differ from normal skin immune reactions. Unfortunately, technical limitations prevented us from analyzing dynamics of vedolizumab target cells in the transplanted abdominal wall as only formalin-fixed paraffin-embedded tissue was available, while the currently available antibody against α4β7 only works on frozen tissue. There is clinical evidence in psoriasis and graft-versus-host disease of the skin that points to an indirect systemic effect of vedolizumab that might help explain the effects observed on the AWTx.29,30
Although these hypotheses cannot be substantiated within this case report, new studies in vedolizumab-treated patients should be focused on α4β7+ upregulation in different cell activation processes (alloreactivity, autoimmunity, infection), the use of alternative cell migration routes in these situations and alternative mechanisms of action as suggested in IBD patients.23 Furthermore, vedolizumab should be studied in more patients undergoing ITx without the history of inflammatory disease.25,31
In conclusion, we present an observational study of a unique case with successful treatment of ACR of an intestinal and abdominal wall graft which was safe and did not hamper the clearance of an astrovirus and primary CMV infections. Our analyses on the dynamics vedolizumab targets and Treg suggest that α4β7+ cells do play a role in ACR but that cell migration to the gut can also use alternative routes and/or that vedolizumab has additional mechanisms of action. This unique case taught us that vedolizumab can be considered as safe treatment option for treating ACR in patients who failed conventional treatment thereby adding a treatment option improving graft survival in ITx.
ACKNOWLEDGMENTS
TAKEDA Pharmaceutical Company Ltd. supplied the Act-1 antibody for immunohistochemistry. We would like to thank Dr Andreas Pascher for providing us with valuable information on his patient from the Charité – Universitätsmedizin hospital, Berlin.
Footnotes
Published online 17 January, 2020.
This study was partially sponsored by an Investigator-Initiated Research Grant from TAKEDA Pharmaceutical Company Ltd.
The authors declare no conflicts of interest.
G.T. participated in study design; data acquisition, analysis, and interpretation; manuscript drafting and revision; and final approval. G.K.-U. participated in data analysis and interpretation, manuscript writing and revision, and final approval. T.B. participated in data acquisition and analysis, manuscript revision, and final approval. G.F.H.D. participated in data interpretation, manuscript revision, and final approval. J.W.H. participated in data interpretation, manuscript revision, and final approval. K.N.F. participated in data interpretation, manuscript writing and revision, and final approval. G.D. participated in study concept and design, data analysis and interpretation, manuscript revision, and final approval.
REFERENCES
- 1.Amin A, Farmer DG. Current outcomes after pediatric and adult intestinal transplantation. Curr Opin Organ Transplant. 2019; 242193–198 [DOI] [PubMed] [Google Scholar]
- 2.Smith JM, Weaver T, Skeans MA, et al. OPTN/SRTR 2016 annual data report: intestine. Am J Transplant. 2018; 18Suppl 1254–290 [DOI] [PubMed] [Google Scholar]
- 3.Trentadue G, Dijkstra G. Current understanding of alloimmunity of the intestinal graft. Curr Opin Organ Transplant. 2015; 203286–294 [DOI] [PubMed] [Google Scholar]
- 4.Zuber J, Shonts B, Lau SP, et al. Bidirectional intragraft alloreactivity drives the repopulation of human intestinal allografts and correlates with clinical outcome. Sci Immunol. 2016; 14eaah3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz P, Bagni A, Brown R, et al. Histological criteria for the identification of acute cellular rejection in human small bowel allografts: results of the pathology workshop at the VIII international small bowel transplant symposium. Transplant Proc. 2004; 362335–337 [DOI] [PubMed] [Google Scholar]
- 6.Kroemer A, Cosentino C, Kaiser J, et al. Intestinal transplant inflammation: the third inflammatory bowel disease. Curr Gastroenterol Rep. 2016; 181156. [DOI] [PubMed] [Google Scholar]
- 7.Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol. 2009; 407909–917 [DOI] [PubMed] [Google Scholar]
- 8.Gorfu G, Rivera-Nieves J, Ley K. Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med. 2009; 97836–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ihara Y, Miyagawa S, Hasegawa T, et al. Effect of blocking the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in a rat small intestinal transplantation model. Transpl Immunol. 2007; 174271–277 [DOI] [PubMed] [Google Scholar]
- 10.Poole RM. Vedolizumab: first global approval. Drugs. 2014; 74111293–1303 [DOI] [PubMed] [Google Scholar]
- 11.Boland BS, Riedl MA, Valasek MA, et al. Vedolizumab in patients with common variable immune deficiency and gut inflammation. Am J Gastroenterol. 2017; 112101621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cushing KC, Mino-Kenudson M, Garber J, et al. Vedolizumab as a novel treatment for refractory collagenous colitis: a case report. Am J Gastroenterol. 2018; 1134632–633 [DOI] [PubMed] [Google Scholar]
- 13.Kim HP, Reed CC, Herfarth HH, et al. Vedolizumab treatment may reduce steroid burden and improve histology in patients with eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2018; 16121992–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarini AA, Hruz P, Berger CT, et al. Vedolizumab as a successful treatment of CTLA-4-associated autoimmune enterocolitis. J Allergy Clin Immunol. 2017; 13931043–1046.e5 [DOI] [PubMed] [Google Scholar]
- 15.Rosario M, Dirks NL, Milch C, et al. A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of vedolizumab. Clin Pharmacokinet. 2017; 56111287–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeissig S, Rosati E, Dowds CM, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut. 2019; 68125–39 [DOI] [PubMed] [Google Scholar]
- 17.Pascher A, Atanasov G. The role of biologicals in intestinal transplantation. Curr Opin Organ Transplant. 2016; 212171–177 [DOI] [PubMed] [Google Scholar]
- 18.Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008; 871396–1400 [DOI] [PubMed] [Google Scholar]
- 19.Hofker TO, Kaijser MA, Nieuwenhuijs VB, et al. Distal duodenogastrostomy or proximal jejunogastrostomy in the management of ultra-short bowel. J Gastrointest Surg. 2018; 223538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haveman JW, Tempelman TM, Hofker HS, et al. [First combined intestinal and abdominal wall transplantation in the Netherlands]. Ned Tijdschr Geneeskd. 2016; 160: A9788. [PubMed] [Google Scholar]
- 21.Schippers A, Muschaweck M, Clahsen T, et al. Β7-integrin exacerbates experimental DSS-induced colitis in mice by directing inflammatory monocytes into the colon. Mucosal Immunol. 2016; 92527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lord JD, Long SA, Shows DM, et al. Circulating integrin alpha4/beta7+ lymphocytes targeted by vedolizumab have a pro-inflammatory phenotype. Clin Immunol. 2018; 193: 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lange KM, Moutsianas L, Lee JC, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017; 492256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beduschi T, Garcia J, Jebrock J, et al. Vedolizumab for the treatment of refractory severe rejection in intestinal transplantation [abstract 320.3]. Transplantation. 2017; 1016S2S59 [Google Scholar]
- 25.Norsa L, Joly F, Busch A, et al. Vedolizumab after intestinal transplantation [abstract 1a.131]. Transplantation. 2017; 1016S2S116 [Google Scholar]
- 26.Rawa-Gołębiewska A, Lenarcik M, Zagórowicz E. Resolution of CMV infection in the bowel on vedolizumab therapy. J Crohns Colitis. 2019; 1391234–1235 [DOI] [PubMed] [Google Scholar]
- 27.Kas-Deelen AM, Bakker WW, Olinga P, et al. Cytomegalovirus infection increases the expression and activity of ecto-atpase (CD39) and ecto-5’nucleotidase (CD73) on endothelial cells. FEBS Lett. 2001; 4911-221–25 [DOI] [PubMed] [Google Scholar]
- 28.Gerlach UA, Vrakas G, Sawitzki B, et al. Abdominal wall transplantation: skin as a sentinel marker for rejection. Am J Transplant. 2016; 1661892–1900 [DOI] [PubMed] [Google Scholar]
- 29.Belvis Jiménez M, Maldonado Pérez B, Argüelles-Arias F. Using vedolizumab to treat severe Sweet’s syndrome in a patient with ulcerative colitis. J Crohns Colitis. 20181–2 [DOI] [PubMed] [Google Scholar]
- 30.Sody E, Körber A. Psoriasis induced by vedolizumab. Inflamm Bowel Dis. 2017; 232E9–E11 [DOI] [PubMed] [Google Scholar]
- 31.Fløisand Y, Lundin KEA, Lazarevic V, et al. Targeting integrin α4β7 in steroid-refractory intestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2017; 231172–175 [DOI] [PubMed] [Google Scholar]


