Abstract
Nuclear factor-κB-inducing kinase (NIK) is a new regulator of nuclear factor-κB signaling, which plays an important role in tumorigenesis. This study aimed to examine the expression of NIK in gastric cancer and investigate its clinical significance.
Tumor issues were collected from 80 gastric cancer patients who received surgery and the diagnosis was confirmed by postoperative pathological analysis. The expression of NIK in gastric cancer tissues and adjacent normal mucosa was detected by immunohistochemical analysis. The associations between NIK expression and clinicopathological features of the patients were further analyzed.
NIK expression was significantly higher in gastric cancer tissues than in adjacent normal tissues (P < .05). Furthermore, NIK expression showed significant association with UICC stage, T status, and differentiation, but not with age and gender of gastric cancer patients.
NIK is overexpressed in gastric cancer and is a potential diagnostic marker of gastric cancer.
Keywords: diagnosis, gastric cancer, marker, Nuclear factor-κB-inducing kinase
1. Introduction
Gastric cancer remains one of the leading causes of cancer related deaths worldwide.[1] Despite the advances in cancer treatment in the past decades, the survival of patients with gastric cancer remains poor, and it is urgent to develop novel diagnostic and therapeutic approaches for gastric cancer.
Nuclear factor κB (NF-κB) is a transcription factor that plays an important role in immune response, inflammation, and tumorigenesis.[2] Canonical NF-κB pathway is initiated by the activation of inhibitor of κB (IκB) kinase (IKK) β, but does not involve IKKα.[3] In contrast, the activation of noncanonical NF-κB pathway depends on NF-κB-inducing kinase (NIK) and IKKα, but does not involve IKKβ.[4]
A variety of genetic and epigenetic changes could contribute to tumorigenesis.[5,6] However, constitutive NF-κB activation has been detected in some cancers including gastric cancer.[7] Therefore, the dysregulation of NF-κB signaling is crucially involved in constitutive NF-κB activation in these cancers. In this study, we focused on NIK as an important regulator of NF-κB signaling, and compared the expression of NIK in tumor tissues and adjacent normal mucosa of gastric cancer patients. Moreover, we investigated the clinical significance of NIK in gastric cancer patients.
2. Materials and methods
2.1. Patients
Total 80 patients were included in this study who underwent surgical resection of gastric cancer at our hospital. The tumors were staged according to the guidelines of UICC. All patients did not receive neoadjuvant chemotherapy or radiotherapy before the surgery. This study was approved by Ethics Committee of The Affiliated Huai’an No.1 People's Hospital and all patients signed informed consents before the study.
2.2. Immunohistochemistry
Freshly resected gastric cancer and adjacent normal mucosa tissues were fixed in 10% formalin, embedded in paraffin, and then cut into serial sections (2 μm). Next, the sections were deparaffinized, hydrated, received antigen retrieval, incubated with peroxidase blocking solution at room temperature for 10 minutes, blocked with normal goat serum at room temperature for 10 minutes, and then incubated with NIK antibody (Santa Cruz Biotech, Santa Cruz, CA) at room temperature for 1 hour. Next, the sections were washed and incubated with biotinylated secondary antibody (Boshide Bio, Wuhan, China) at room temperature for 1 hour, followed by incubation with streptavidin-peroxidase and chromogenic reagent. Finally, the sections were washed and counterstained with hematoxylin and eosin. Primary antibody was replaced with saline as negative control.
For each section, at least three fields at high magnification (400 ×) were randomly selected in a double blinded manner. The staining was considered positive if brownish-yellow grains were observed in the cytoplasm. The percentage of positive cells was interpreted as follows: 0% to 5%, negative; 6% to 25%, weakly positive; 26% to 75%, moderately positive; and ≥76%, strongly positive. Weakly, moderately, and strongly positive were defined as positive staining.
2.3. Statistical analysis
All data were analyzed by using SPSS 22.0 software (SPSS Inc., Chicago, IL). The comparison of the groups was made by using Student t test or Fisher exact test. P < .05 was considered significant.
3. Results
3.1. Expression of NIK protein in gastric cancer tissues
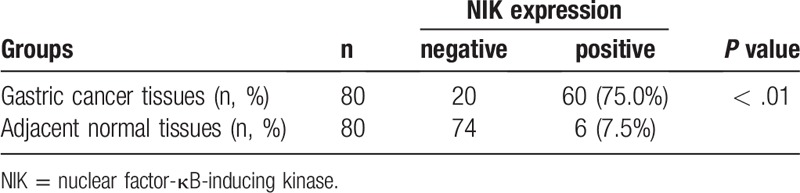
IHC analysis showed negative staining of NIK in adjacent normal mucosa tissues and positive staining of NIK in gastric cancer tissues. In gastric cancer tissues, NIK staining was very strong in the cytoplasm as brown grains (Fig. 1). Moreover, positive rate of NIK staining was 74.3% in NSCLC tissues, significant higher than 6.9% in normal tissues (P < .01, Table 1).
Figure 1.
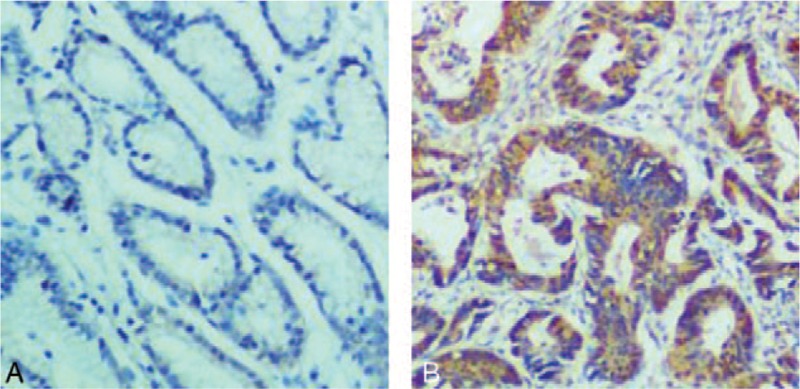
Immunohistochemical analysis of NIK expression in gastric cancer tissues. Shown were the representative images. A. Negative staining of NIK in adjacent normal mucosa tissues. B. Strong staining of NIK in gastric cancer tissues. Original amplification: ×400. NIK = nuclear factor-κB-inducing kinase.
Table 1.
Comparison of NIK expression in gastric cancer tissues and adjacent normal mucosa tissues.
3.2. Correlation of NIK expression with clinicopathological features of gastric cancer
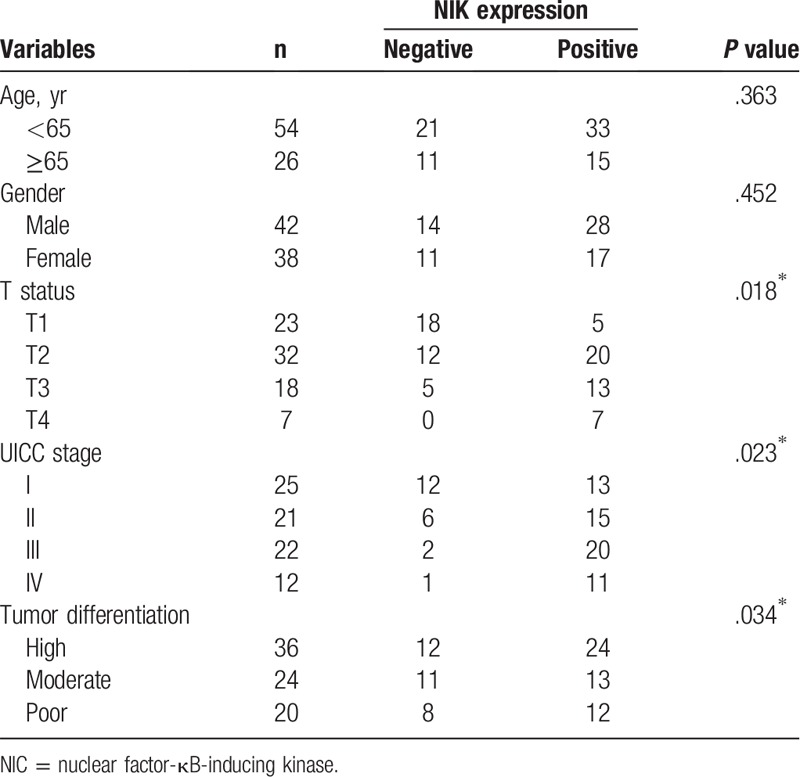
Next we analyzed the correlation of NIK expression with clinicopathological features of gastric cancer patients. We found that positive NIK expression was significantly associated with T status, clinical stage and tumor differentiation, but not with age and gender of gastric cancer patients (P < .05, Table 2).
Table 2.
Correlation of NIK expression with clinicopathologic characteristics of gastric cancer.
4. Discussion
In this study we reported that NIK expression was significantly higher in gastric cancer tissues than in adjacent normal mucosa tissues. Moreover, we demonstrated that NIK expression was significantly associated with T status, clinical stage and tumor differentiation of gastric cancer, indicating the important role of NIK in gastric cancer.
NF-κB signaling is crucially implicated in cancer development, progression and metastasis, and emerges as a potential target for cancer therapy.[8,9] NIK is a key factor to promote the activation of NF-κB signaling. Consistently, increased NIK expression has been reported in various tumor tissues such as glioma, melanoma, and prostate cancer.[10] In addition, the inhibition of NIK expression reduced tumor cell proliferation and promoted tumor cell apoptosis.[11] These results suggest that NIK plays an oncogenic role and is a potential target for cancer treatment.[12] In this study, we reported higher expression of NIK in gastric cancer tissues than in adjacent normal mucosa tissues. Furthermore, we analyzed the clinical significance of NIK. The results showed that positive NIK expression was significantly associated with T status, clinical stage and tumor differentiation of gastric cancer. These data indicate that NIK expression is upregulated and promotes gastric cancer, but further studies are needed to examine the prognostic value of NIK in gastric cancer.
In conclusion, although this study has several limitations such as limited sample size and the lack of mechanistic study on the function of NIK in NSCLC, our results suggest that NIK is overexpressed in gastric cancer and is a novel diagnostic marker of NSCLC.
Author contributions
Conceptualization: Jiaxin Li.
Investigation: Liang Xue, Yuexia Wang, Xian Ding, Hairong Teng.
Footnotes
Abbreviations: NF-κB = nuclear factor κB, NIK = nuclear factor-κB-inducing kinase.
How to cite this article: Teng H, Xue L, Wang Y, Ding X, Li J. Nuclear factor κB -inducing kinase is a diagnostic marker of gastric cancer. Medicine. 2020;99:5(e18864).
HT and LX These authors contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 2009;27:693–733. [DOI] [PubMed] [Google Scholar]
- [3].Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol 2011;12:695–708. [DOI] [PubMed] [Google Scholar]
- [4].Senftleben U, Cao Y, Xiao G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001;293:1495–9. [DOI] [PubMed] [Google Scholar]
- [5].Tian H, Cong P, Qi R, et al. Decreased invasion ability of hypotaurine synthesis deficient glioma cells was partially due to hypomethylation of Wnt5a promoter. Biocell 2017;41:27–32. [Google Scholar]
- [6].Wong ET, Tergaonkar V. Roles of NF-kappaB in health and disease: mechanisms and therapeutic potential. Clin Sci 2009;116:451–65. [DOI] [PubMed] [Google Scholar]
- [7].Peloponese JM, Yeung ML, Jeang KT. Modulation of nuclear factor-kappaB by human T cell leukemia virus type 1 Tax protein: implications for oncogenesis and inflammation. Immunol Res 2006;34:1–2. [PubMed] [Google Scholar]
- [8].Soltanian S, Riahirad H, Pabarja A, et al. Kaempferol and docetaxel diminish side population and down-regulate some cancer stem cell markers in breast cancer cell line MCF-7. Biocell 2017;41:33–40. [Google Scholar]
- [9].Tong L, Yuan Y, Wu S. Therapeutic microRNAs targeting the NF-kappa B signaling circuits of cancers. Adv Drug Deliv Rev 2015;81:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Noort AR, van Zoest KP, Weijers EM, et al. NF-kappaB-inducing kinase is a key regulator of inflammation-induced and tumour-associated angiogenesis. J Pathol 2014;234:375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thu YM, Su Y, Yang J, et al. NF-kappaB inducing kinase (NIK) modulates melanoma tumorigenesis by regulating expression of pro-survival factors through the beta-catenin pathway. Oncogene 2012;31:2580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Storz P. Targeting the alternative NF-kappaB pathway in pancreatic cancer: a new direction for therapy? Expert Rev Anticancer Ther 2013;13:501–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


