Supplemental Digital Content is available in the text
Keywords: Alzheimer disease, amyotrophic lateral sclerosis, association, Parkinson disease, R47H, triggering receptors expressed on myeloid cells 2
Abstract
Background:
Recent studies have suggested that the potential functional polymorphism R47H in triggering receptors expressed on myeloid cells 2 (TREM2) is associated with several neurodegenerative diseases, however, the results remain inconclusive. This meta-analysis aimed to investigate the association between TREM2 R47H and the risk for 3 typical neurodegenerative diseases: Alzheimer disease (AD), Parkinson disease (PD), and amyotrophic lateral sclerosis (ALS).
Methods:
A literature review was carried out using PubMed, Medline, and Embase. Data analysis was conducted using Stata 15.0 software. The pooled odds ratio (ORs) and 95% confidence interval (CIs) were calculated.
Results:
A total of 35 articles were identified as eligible: 22 on AD, 3 on ALS, 7 on PD, 2 on AD and ALS, and 1 on ALS and PD. The AD set included 23,092 cases and 30,920 controls, the ALS set included 7391 cases and 12,442 controls, and the PD set included 8498 patients and 9161 controls. We found that R47H was associated with an increased risk of AD in the total pooled population (P < .001, OR = 4.02, 95% CI = 3.15–5.13). However, this significant difference existed for Caucasian people (OR = 4.16, 95% CI = 3.24–5.33) but not for Asian or African people. Moreover, we did not find any significant differences in minor allele frequency distribution between the PD and control groups or between the ALS and control groups, not only for the total pooled population but also for the subgroups of different ethnicities.
Conclusion:
Our study suggested that R47H in the TREM2 gene leads to an increased risk for developing AD, but not for ALS and PD, which adds evidence to the notion that diverse pathogenesis may be involved in different neurogenerative diseases.
1. Introduction
Neurodegenerative diseases are characterized by a progressive loss of neuron cells in the particular regions of the brain that are correlated with each disease's symptoms. These diseases include Alzheimer disease (AD), frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), Parkinson disease (PD), and many others. In AD, this means a loss of neurons in the hippocampus and/or cortex; in ALS, motor neurons are lost, while in PD, there is a degeneration of dopaminergic neurons. These various types of damage lead to differing manifestations of motor and nonmotor symptoms.[1] However, the mechanisms that lead to neurodegeneration remain unclear.
Gene defects are prominent factors in both the etiology and pathogenesis of neurodegenerative diseases. In previous studies, gene mutations resulted in AD, ALS, and PD in only a small group of patients. In addition, hundreds of genetic variants located in dozens of genes have been associated with susceptibility to such diseases.[2] While the majority of these susceptible genes do not overlap across diseases, some mutations in certain genes, such as TAR DNA-binding protein 43 (TDP43), which was first reported in ALS,[3] or progranulin (PGRN), initially identified in FTD,[4] have been linked to other neurodegenerative diseases.[5] The rare missense variant p.R47H (rs75932628) of triggering receptors expressed on myeloid cells 2 (TREM2), which is a surface receptor expressed on myeloid cells, has been reported to be associated with a risk of sporadic late-onset AD for Spanish,[6] French,[7] British,[8] Portuguese, and American people.[7,9] However, no association between TREM2 R47H and AD has been found in people from the UK,[10] Belgium,[11] or Iran[12] in different cohorts of replication studies. Moreover, various studies with Asian people, including 4 from China[13–16] and 1 from Korea,[17] have also failed to find the R47H variant in 5 cohorts of 2958 cases and 3358 controls. In Japan, while 3 subjects carrying R47H were reported, no significant association was found between this variant and AD.[18]
The R47H variant, which is located in exon 2 of TREM2, has been suggested to play a key role in neurodegenerative diseases due to its function in regulating cell numbers and phagocytosis, in controlling synaptic pruning, in monitoring synaptic function, and especially in modulating inflammatory responses.[19] As a result of these important functions, the variant has also been investigated in other neurodegenerative diseases, such as FTD,[20] ALS, PD, multiple system atrophy (MSA),[21] dementia with Lewy bodies (DLB),[22] and essential tremor (ET).[23] In MSA, DLB, or ET, only 1 or 2 studies have investigated the association between R47H in TREM2 and each disease, and either no association or a marginally significant association was found.[21–23] In AD, FTD, ALS, and PD, at least 5 independent case–control studies have explored the association between the R47H variant in TREM2 and susceptibility for each disease. However, inconsistent or indefinite correlations between this variant and disease risks were found for AD, ALS, and PD, although a recent meta-analysis found an increasing disease risk for developing FTD.[20]
As mentioned above, limited numbers of participants were included in each study. Additionally, the differing ethnicities of participants may contribute to this picture of inconsistent or conflicting results, especially for a variant in which risk allele is rare. We therefore carried out a meta-analysis and systematic review that aimed to investigate a more precise description of the relationship between the R47H variant of TREM2 and the risk of developing AD, ALS, and MSA by pooling 47 case–control studies from a total of 35 published articles.
2. Methods
2.1. Literature search
To identify all articles that examined the association of TREM2 polymorphisms with these 3 neurodegenerative diseases, 2 researchers independently conducted a literature search using the PubMed, Embase, and Medline databases (from January 2013 to November 15, 2019) using the keywords “TREM2 or triggering receptor expressed on myeloid cells 2,” “polymorphism or R47H or rs75932628” PLUS “Alzheimer disease or AD” OR “Parkinson disease or PD” OR “amyotrophic lateral sclerosis or ALS.” Once the articles had been gathered, reference lists were examined manually to further identify potentially relevant studies.
The R47H polymorphism includes “T” and “C” alleles. T is minor and is taken as the high-risk allele, while C is the lower-risk allele. The following analyses are based on the allelic genetic model, which can be described as the T allele versus the C allele.
2.2. Inclusion and exclusion criteria
Studies had to meet the following criteria to be eligible: evaluate the association between the R47H variant of TREM2 and 1 of the 3 neurodegenerative diseases involved in this study; follow an unrelated case–control study design, meaning that if studies had partly overlapping participants, only the study with a larger sample was selected; measure available genotype frequency in case and control groups plus sufficient data for estimating an odds ratio (OR) with 95% confidence interval (CI); and have genotype frequencies in the control group that were consistent with the Hardy–Weinberg equilibrium (HWE).
Studies were excluded if they had one or more of the following factors: the design was based on family, sibling pairs, or case only; the genotype/allele frequency of R47H of TREM2 was neither reported nor available; there was insufficient information for the extraction of data; or the R47H variant of TREM2 deviated from HWE in the control group.
2.3. Data extraction
All data were extracted independently by 2 authors (BZ and RL) following the criteria listed above. For each study, the following information was extracted: the name of the first author, publication year, the ethnicity (country) of the sample, sample collection area, genotyping methods, sample size (numbers of both cases and controls), types of neurodegenerative disease, genotype frequency, minor allele frequency, P value, OR (95% CI), age range, and sex ratio (see Table 1 and Supplementary Table 1).
Table 1.
Principal characteristics of studies included in the meta-analysis.

2.4. Statistical analysis
Statistical analysis was carried out using STATA 15.0 (Stata Corp LP, College Station, TX). The association between TREM2 R47H and the 3 neurodegenerative diseases was measured by calculating pooled ORs and 95% CIs. The significance of the pooled OR was measured using the Z test. The risk of R47H in neurodegenerative disease was evaluated through comparison with the reference wild allele C. Heterogeneity between individual studies was tested using Cochran (Q) chi-squared test, which is a weighted sum of the squares of the standard deviations of individual OR estimates from the overall estimation. The alpha level was set to 0.10. If the Q statistic was significant (PQ < .10), a random-effects model was assumed; otherwise, a fixed-effects model was employed. We utilized a forest plot to graphically present the pooled ORs and 95% CIs. Each study was represented by a square in the plot, and the weight of each study was also shown. To evaluate ethnicity-specific effects, a subgroup analysis was performed according to the ethnicity of each study population. Publication bias was detected using Egger test. Additionally, Egger funnel plots were drawn. Sensitivity analysis was carried out to assess the potential influences of any single study on the pooled ORs. P < .05 was considered statistically significant.
2.5. Ethical review
Ethical or institutional review board approval was not required for this meta-analysis since data were extracted from previously published studies.
3. Results
3.1. Characteristics of studies
A total of 505 abstracts were retrieved by our search for “TREM2 or triggering receptor expressed on myeloid cells 2,” “polymorphism or R47H or rs75932628” PLUS “Alzheimer disease or AD” OR “Parkinson disease or PD” OR “Amyotrophic Lateral Sclerosis or ALS.” From these, 35 articles that met the inclusion criteria were found. A flow chart for the selection of studies and reasons for exclusion is shown in Fig. 1.
Figure 1.
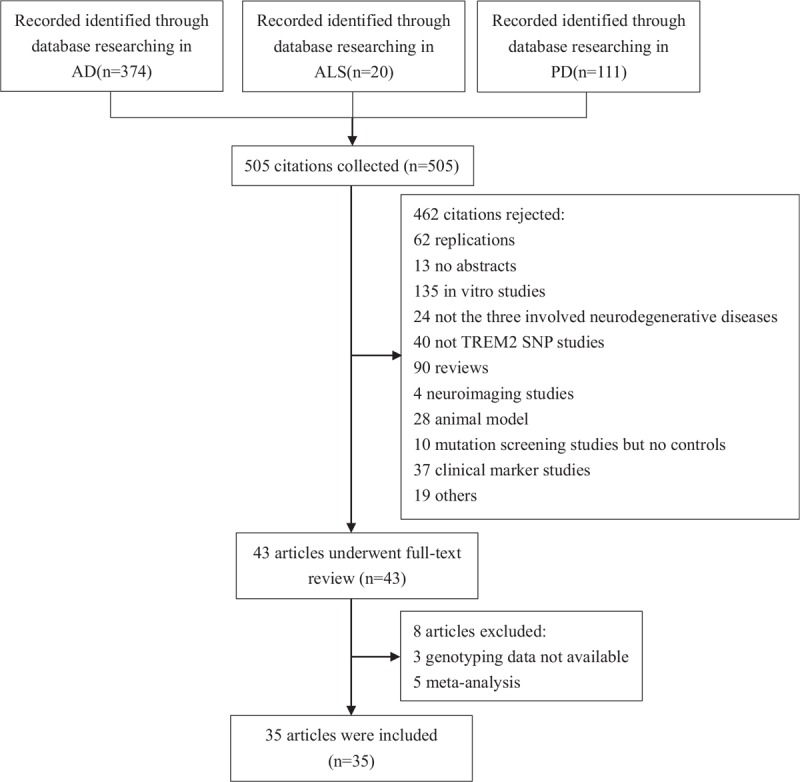
Flow chart of the electronic search strategy for meta-analysis.
Aside from 2 articles[24,25] that included participants living with AD and ALS and another[26] that included those with ALS and PD, the remaining 32 articles investigated just 1 disease. Twenty-four articles,[6–18,24,25,27–35] comprising 25 studies about AD, were included in our meta-analysis (23,092 cases and 30,920 controls), as well as 6 articles that comprised 9 studies on ALS (7391 cases, 12,442 controls)[24–26,36–38] and 8 articles that comprised 13 studies on PD (8498 cases, 9161 controls).[26,37,39–44] There were 35 studies with Caucasian people, 11 studies with Asian people, and 2 with African people. Detailed characteristics for each study in the meta-analysis can be seen in supplementary Table 1 and Table 1.
3.2. Quantitative synthesis
The Q test and I2 statistics were used to examine heterogeneity. Fixed-effects models were used for AD and PD (PQ > .10), while random-effects models were used for ALS (PQ < .10). The results of the meta-analysis on the association between R47H and the risk of developing neurodegenerative diseases are shown in Table 2. Pooled results showed that the T allele was associated with an increased risk of AD (OR, 4.02; 95% CI, 3.15–5.13; P < .001). When studies were divided according to genetic background, the results indicated that significant associations were observed in Caucasian people (OR, 4.16; 95% CI, 3.24–5.33; P < .001) but not in Asian or African people (Fig. 2 A). Moreover, marginally significant differences in the minor allele frequency distribution between TREM2 R47H and PD in the pooled and European subgroups were found (P = .063, P = .073, respectively) but were not found in the Asian subgroup (Fig. 2 C). However, there was no significant difference found between the R47H variant and ALS, not only in the pooled but also in the ethnicity-based analysis (Fig. 2 B).
Table 2.
Summary risk estimates for association between R47H of TREM2 and 3 neurodegenerative diseases.

Figure 2.

The forest plots of OR and 95% CI for TREM2 variant R47H in 3 neurodegenerative diseases. Meta-analyses of data sets assessing the association between TREM2 R47H and Alzheimer disease (AD; A), amyotrophic lateral sclerosis (ALS; B), and Parkinson disease (PD; C). The x-axis depicts the odds ratio (OR). Study-specific ORs (black diamond) and 95% confidence intervals (CI, lines) were calculated using an allelic model. A fixed-effect meta-analysis was calculated in AD and PD; a random-effect model was used in ALS. I2 is an estimate of the amount of heterogeneity that is beyond chance. TREM2 = triggering receptors expressed on myeloid cells 2.
3.3. Assessment of potential publication bias
Both funnel plots and Egger test were performed to assess the publication bias of the included studies. All values of Egger test were >0.10, indicating that there was no statistical evidence for publication bias among these studies. Funnel plots for the meta-analysis of the TREM2 R47H variant in the studies under the allelic genetic model appeared symmetrical in all diseases and can be seen in Fig. 3.
Figure 2 (Continued).

The forest plots of OR and 95% CI for TREM2 variant R47H in 3 neurodegenerative diseases. Meta-analyses of data sets assessing the association between TREM2 R47H and Alzheimer disease (AD; A), amyotrophic lateral sclerosis (ALS; B), and Parkinson disease (PD; C). The x-axis depicts the odds ratio (OR). Study-specific ORs (black diamond) and 95% confidence intervals (CI, lines) were calculated using an allelic model. A fixed-effect meta-analysis was calculated in AD and PD; a random-effect model was used in ALS. I2 is an estimate of the amount of heterogeneity that is beyond chance. TREM2 = triggering receptors expressed on myeloid cells 2.
Figure 3.

Egger funnel plot for publication bias analysis for TREM2 variant R47H. A: Egger test for AD; B: Egger test for ALS; C: Egger test for PD. AD = Alzheimer disease, ALS = amyotrophic lateral sclerosis, PD = Parkinson disease, TREM2 = triggering receptors expressed on myeloid cells 2.
3.4. Sensitivity analysis
A sensitivity analysis was conducted to evaluate the stability of the results. The omission of any study made no significant difference in the association between the R47H variant in TREM2 and the 3 neurodegenerative diseases (supplementary Figure 1), suggesting that our results are statistically robust.
4. Discussion
This meta-analysis provides a systematic evaluation of the roles of TREM2 R47H in susceptibility to the 3 representative neurodegenerative diseases under investigation. We found that R47H in TREM2 increased the risk for AD in Caucasian people but not Asian or African people. However, this variant was not associated with a risk of developing ALS or PD.
TREM2 is expressed on many cells of the myeloid lineage, such as dendritic cells and osteoclasts, as well as bone marrow- and monocyte-derived macrophages. The majority of evidence suggests that TREM2 is expressed within the brain exclusively by microglia.[19] However, TREM2 expression in the central nervous system can also be seen to be regulated throughout development and displays a different expression pattern across different brain regions, such as early elevated expression in specific brain regions, and more expression in the white matter, hippocampus, and spinal cord than in other brain regions[19]; a high density of microglia is also suggested in these regions.
TREM2 regulates myeloid cell numbers, meaning that TREM2 deficiency was shown to prevent increases in brain myeloid cell populations in response to injury and disease. In addition, TREM2 improves myeloid cell survival, proliferation, and differentiation; regulates phagocytic function; and modulates inflammatory responses.[19] All of this suggests a crucial role of TREM2 in neuroimmunology and neuroinflammation. Additionally, other studies have suggested additional roles for TREM2, such as the regulation of synaptic pruning and the monitoring of synaptic function.[45] However, whether TREM2 was the cause of the pathological hallmark of neurodegenerative diseases or was activated by the pathological alterations remains unknown.
Variants in TREM2, such as R47H, R62H, D87N, and L211P, have been extensively found to be associated with neurodegenerative diseases.[19] Among them, R47H, a nonsynonymous substitution, was reported in many neurodegenerative diseases, such as AD, FTD, PD, ALS, MSA, DLB, and ET. Functionally, a recent study found that TREM2 carrying R47 could function to position elements of the ligand-binding surface. However, a disruption of receptor oligomerization by the R47H mutation led to ligand-induced clustering in receptor signaling as well as a reduction in soluble TREM2 levels.[46] More directly, TREM2 has an immune-mediated link to clean up Aβ aggregates by appearing to be capable of mediating Aβ internalization, while Aβ oligomers induce nuclear factor of activated T-cell (NFAT) signaling. However, R47H has been shown to reduce both Aβ internalization and downstream NFAT signaling activity in response to Aβ42.[47] Additionally, the impaired splicing and reduced TREM2 mRNA and protein by R47H confers a loss-of-function-like phenotype in AD, including a reduced density of TREM2 around plaques and increased plaque-associated neuritic dystrophy in mice.[48] Evidence therefore suggests that the R47H polymorphism is a functional variant associated with AD.
In this review, a meta-analysis of 24 articles comprising 25 studies on the association between R47H and AD led to a large sample size. Consistent with previous functional studies, we found that the R47H variant of TREM2 increased the risk of AD in Caucasian people but not in Asian or African people. Undeniably, the minor allele frequency (MAF) of the R47H TREM2 variant is very low, at approximately 0.26% (172/66274) in European people and 0.09% (9/10216) in African people, and is almost absent (0/8614) in Asian people (Exome Aggregation Consortium database). Therefore, studies with large sample sizes are crucial. In 11 of 17 studies with Caucasian people, the total sample sizes for each study were >2000. In contrast, with 2 studies,[11,29] 9 studies consistently found that this variant increased the risk for AD,[6–9,30–32] which was also seen in the heterogeneity tests (Fig. 2 , I2 = 0.0%, P = .804). For Caucasian people, the MAF is approximately 0.80% (270/33,782) in AD patients but is 0.20% (92/44,908) in control groups.
Our analyses represent the most comprehensive assessment of this issue and provide strong statistical evidence that the presence of the rare nonsynonymous variant R47H in TREM2 increases the risk for AD by approximately 4.16-fold in Caucasians. This OR is larger than that estimated in earlier analyses (OR ∼3.17),[37,49,50] which were based on smaller studies. While the total sample size of the AD group and control group in Asian people from 6 studies was in excess of 10,000,[13–18] the MAFs were not significantly different between AD patients and controls in Asian people, at 0.01% (1/10,260) and 0.02% (2/11,674), respectively. This result is consistent with the findings of another meta-analysis, which stated that R47H was not associated with AD in people from Asia or East Asia.[49,50] Although a recent study comprehensively analyzed the association between R47H and AD, with the cohort from Africa pooled into the Caucasian cohort,[50] we initially conducted a meta-analysis that included 2 studies on the association between R47H and AD in African people,[33,34] but this variant did not affect the AD risk for this population (0.09% [2/2142] in patients, 0.06% [3/5255] in controls). Therefore, for this variant, specific ethnicities appear to contribute to disease susceptibility.
Contrasting with the significant results observed for AD, our analyses, which combine recent and previously reported available association data for people living with PD, do not provide convincing statistical support for a role of the R47H variant in TREM2 contributing to the risk of this disease, although an increase toward risk was found. In this meta-analysis of 9 studies, including 6402 PD patients and 7150 controls in Caucasian people,[26,37,39,40] no significant difference in the MAF distribution was found, which is consistent with the finding that it was also not associated with PD in northern European people but is inconsistent with the 3.88-fold increased risk for PD in non-northern European people found by another meta-analysis.[51] Alternatively, another meta-analysis identified that R47H increased the risk for PD by 3.59-fold in North American people but not in Europeans.[50] However, the subgroup analysis methods in these studies remain open to question,[51] as there seems to be no obvious difference in genetic background between northern European and non-northern European people or between North Americans and Europeans. In addition, the study from China included in the non-northern European group was not reasonable.[51] In the current meta-analysis, only 1 (1/9) study whose weight was 17% found a variant increase in the risk for PD (OR: 2.92, 95% [1.04–8.22]).[26] Therefore, no association between this variant and PD in Caucasian people could be accepted. In the ethnicity-specific analysis, while 4557 Asian people, including 2546 people with PD and 2011 controls, were analyzed,[41–44] only 1 PD patient carrying this variant was identified. We can therefore see that this variant may not be associated with the risk for PD.
This is the largest comprehensive assessment of the potential role of R47H in TREM2 and ALS to date, since 6 papers comprising 10 studies were included.[24,26,36–38] No significant difference in MAF distribution was found between ALS and controls in the large sample size investigation, which included 7391 cases and 12,442 controls (0.29% [43/14782] and 0.27% [67/24884], respectively). Our results were consistent with the findings from another 2 meta-analyses involving fewer cohorts in which the variant was not found to be associated with ALS.[37,50] While it has been suggested that ALS and FTD may be within the same spectrum of disorders, our findings indicated differing pathogenesis to some degree since the recent meta-analysis found the variant to be associated with susceptibility to FTD in Caucasian people.[20]
In summary, much research has reported on the function of R47H in TREM2, which is linked to the pathogenesis of AD. Our study supports the notion that this variant may be involved in the development of AD, but not of PD and ALS. This, in turn, suggests that diverse pathogenesis may be involved in these different neurogenerative diseases.
Acknowledgments
There are no financial, personal, or other relationships with other people or organizations that could inappropriately influence (bias) the current work.
Author contributions
Data curation: Bin Zhang, Rui Li, Yufan Zhang.
Formal analysis: Bin Zhang.
Investigation: Bin Zhang, Rui Li.
Methodology: Bin Zhang and Xia Gao.
Project administration: Bin Zhang, Xia Gao.
Supervision: Xia Gao.
Writing – original draft: Bin Zhang.
Writing – review & editing: Xia Gao.
Xia Gao orcid: 0000-0003-3284-0138.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AD = Alzheimer disease, ALS = amyotrophic lateral sclerosis, DLB = dementia with Lewy bodies, ET = essential tremor, FTD = frontotemporal dementia, MAF = minor allele frequency, MSA = multiple system atrophy, PD = Parkinson disease, PGRN = progranulin, TDP43 = TAR DNA-binding protein 43, TREM2 = triggering receptors expressed on myeloid cells 2.
How to cite this article: Zhang B, Li R, Zhang Y, Gao X. Differential role of triggering receptors expressed on myeloid cells 2 R47H in 3 neurodegenerative diseases based on a systematic review and meta-analysis. Medicine. 2020;99:5(e18921).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Scrivo A, Bourdenx M, Pampliega O, et al. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol 2018;17:802–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lill CM, Bertram L. Towards unveiling the genetics of neurodegenerative diseases. Semin Neurol 2011;31:531–41. [DOI] [PubMed] [Google Scholar]
- [3].Nakashima-Yasuda H, Uryu K, Robinson J, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 2007;114:221–9. [DOI] [PubMed] [Google Scholar]
- [4].Schafferer S, Khurana R, Refolo V, et al. Changes in the miRNA-mRNA regulatory network precede motor symptoms in a Mouse Model of Multiple System Atrophy: clinical implications. PLoS One 2016;11:e0150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mosca L, Lunetta C, Tarlarini C, et al. Wide phenotypic spectrum of the TARDBP gene: homozygosity of A382T mutation in a patient presenting with amyotrophic lateral sclerosis, Parkinson's disease, and frontotemporal lobar degeneration, and in neurologically healthy subject. Neurobiol Aging 2012;33:1846.e1–4. [DOI] [PubMed] [Google Scholar]
- [6].Ruiz A, Dols-Icardo O, Bullido MJ, et al. Assessing the role of the TREM2 p.R47H variant as a risk factor for Alzheimer's disease and frontotemporal dementia. Neurobiol Aging 2014;35:444.e1–4. [DOI] [PubMed] [Google Scholar]
- [7].Pottier C, Wallon D, Rousseau S, et al. TREM2 R47H variant as a risk factor for early-onset Alzheimer's disease. J Alzheimers Dis 2013;35:45–9. [DOI] [PubMed] [Google Scholar]
- [8].Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer's disease. N Engl J Med 2013;368:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rosenthal SL, Bamne MN, Wang X, et al. More evidence for association of a rare TREM2 mutation (R47H) with Alzheimer's disease risk. Neurobiol Aging 2015;36:2443.e21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Finelli D, Rollinson S, Harris J, et al. TREM2 analysis and increased risk of Alzheimer's disease. Neurobiol Aging 2015;36:546.e9–13. [DOI] [PubMed] [Google Scholar]
- [11].Cuyvers E, Bettens K, Philtjens S, et al. Investigating the role of rare heterozygous TREM2 variants in Alzheimer's disease and frontotemporal dementia. Neurobiol Aging 2014;35:726.e11–9. [DOI] [PubMed] [Google Scholar]
- [12].Mehrjoo Z, Najmabadi A, Abedini SS, et al. Association study of the TREM2 gene and identification of a novel variant in Exon 2 in Iranian patients with late-onset Alzheimer's disease. Med Princ Pract 2015;24:351–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yu JT, Jiang T, Wang YL, et al. Triggering receptor expressed on myeloid cells 2 variant is rare in late-onset Alzheimer's disease in Han Chinese individuals. Neurobiol Aging 2014;35:937.e1–3. [DOI] [PubMed] [Google Scholar]
- [14].Ma J, Zhou Y, Xu J, et al. Association study of TREM2 polymorphism rs75932628 with late-onset Alzheimer's disease in Chinese Han population. Neurol Res 2014;36:894–6. [DOI] [PubMed] [Google Scholar]
- [15].Wang P, Guo Q, Zhou Y, et al. Lack of association between triggering receptor expressed on myeloid cells 2 polymorphism rs75932628 and late-onset Alzheimer's disease in a Chinese Han population. Psychiatr Genet 2018;28:16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jiao B, Liu X, Tang B, et al. Investigation of TREM2, PLD3, and UNC5C variants in patients with Alzheimer's disease from mainland China. Neurobiol Aging 2014;35:2422.e9–11. [DOI] [PubMed] [Google Scholar]
- [17].Chung SJ, Kim MJ, Kim J, et al. Exome array study did not identify novel variants in Alzheimer's disease. Neurobiol Aging 2014;35:1958.e13–4. [DOI] [PubMed] [Google Scholar]
- [18].Miyashita A, Wen Y, Kitamura N, et al. Lack of genetic association between TREM2 and late-onset Alzheimer's disease in a Japanese population. J Alzheimers Dis 2014;41:1031–8. [DOI] [PubMed] [Google Scholar]
- [19].Jay TR, von Saucken VE, Landreth GE. TREM2 in neurodegenerative diseases. Mol Neurodegener 2017;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Su WH, Shi ZH, Liu SL, et al. The rs75932628 and rs2234253 polymorphisms of the TREM2 gene were associated with susceptibility to frontotemporal lobar degeneration in Caucasian populations. Ann Hum Genet 2018;82:177–85. [DOI] [PubMed] [Google Scholar]
- [21].Ogaki K, Heckman MG, Koga S, et al. Association study between multiple system atrophy and TREM2 p.R47H. Neurol Genet 2018;4:e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Walton RL, Soto-Ortolaza AI, Murray ME, et al. TREM2 p.R47H substitution is not associated with dementia with Lewy bodies. Neurol Genet 2016;2:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ortega-Cubero S, Lorenzo-Betancor O, Lorenzo E, et al. TREM2 R47H variant and risk of essential tremor: a cross-sectional international multicenter study. Parkinsonism Relat Disord 2015;21:306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peplonska B, Berdynski M, Mandecka M, et al. TREM2 variants in neurodegenerative disorders in the Polish population. Homozygosity and compound heterozygosity in FTD patients. Amyotroph Lateral Scler Frontotemporal Degener 2018;19:407–12. [DOI] [PubMed] [Google Scholar]
- [25].Ayer AH, Wojta K, Ramos EM, et al. Frequency of the TREM2 R47H Variant in Various Neurodegenerative Disorders. Alzheimer Dis Assoc Disord 2019;33:327–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rayaprolu S, Mullen B, Baker M, et al. TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson's disease. Mol Neurodegener 2013;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Giraldo M, Lopera F, Siniard AL, et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer's disease. Neurobiol Aging 2013;34:2077.e11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Benitez BA, Cooper B, Pastor P, et al. TREM2 is associated with the risk of Alzheimer's disease in Spanish population. Neurobiol Aging 2013;34:1711.e15–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Slattery CF, Beck JA, Harper L, et al. R47H TREM2 variant increases risk of typical early-onset Alzheimer's disease but not of prion or frontotemporal dementia. Alzheimers Dement 2014;10:602.e4–8.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gonzalez Murcia JD, Schmutz C, Munger C, et al. Assessment of TREM2 rs75932628 association with Alzheimer's disease in a population-based sample: the Cache County Study. Neurobiol Aging 2013;34:2889.e11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jin SC, Benitez BA, Karch CM, et al. Coding variants in TREM2 increase risk for Alzheimer's disease. Hum Mol Genet 2014;23:5838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Roussos P, Katsel P, Fam P, et al. The triggering receptor expressed on myeloid cells 2 (TREM2) is associated with enhanced inflammation, neuropathological lesions and increased risk for Alzheimer's dementia. Alzheimers Dement 2015;11:1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jin SC, Carrasquillo MM, Benitez BA, et al. TREM2 is associated with increased risk for Alzheimer's disease in African Americans. Mol Neurodegener 2015;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Landoulsi Z, Ben Djebara M, Kacem I, et al. Genetic analysis of TREM2 variants in Tunisian patients with Alzheimer's disease. Med Princ Pract 2018;27:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Arboleda-Bustos CE, Ortega-Rojas J, Mahecha MF, et al. The p.R47H variant of TREM2 gene is associated with late-onset alzheimer disease in colombian population. Alzheimer Dis Assoc Disord 2018;32:305–8. [DOI] [PubMed] [Google Scholar]
- [36].Cady J, Koval ED, Benitez BA, et al. TREM2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol 2014;71:449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lill CM, Rengmark A, Pihlstrom L, et al. The role of TREM2 R47H as a risk factor for Alzheimer's disease, frontotemporal lobar degeneration, amyotrophic lateral sclerosis, and Parkinson's disease. Alzheimers Dement 2015;11:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen X, Chen Y, Wei Q, et al. Assessment of TREM2 rs75932628 association with amyotrophic lateral sclerosis in a Chinese population. J Neurol Sci 2015;355:193–5. [DOI] [PubMed] [Google Scholar]
- [39].Mengel D, Thelen M, Balzer-Geldsetzer M, et al. TREM2 rare variant p.R47H is not associated with Parkinson's disease. Parkinsonism Relat Disord 2016;23:109–11. [DOI] [PubMed] [Google Scholar]
- [40].Benitez BA, Cruchaga C. United States-Spain Parkinson's Disease Research G. TREM2 and neurodegenerative disease. N Engl J Med 2013;369:1567–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Feng SJ, Nie K, Gan R, et al. Triggering receptor expressed on myeloid cells 2 variants are rare in Parkinson's disease in a Han Chinese cohort. Neurobiol Aging 2014;35:1780.e11–2. [DOI] [PubMed] [Google Scholar]
- [42].Li Z, Zhong L, Gu L, et al. Association study of TREM2 polymorphism rs75932628 with leucoaraiosis or Parkinson's disease in the Han Chinese population. BMJ Open 2016;6:e009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tan T, Song Z, Yuan L, et al. Genetic analysis of TREM2 variants in Chinese Han patients with sporadic Parkinson's disease. Neurosci Lett 2016;612:189–92. [DOI] [PubMed] [Google Scholar]
- [44].Chen Y, Chen X, Guo X, et al. Assessment of TREM2 rs75932628 association with Parkinson's disease and multiple system atrophy in a Chinese population. Neurol Sci 2015;36:1903–6. [DOI] [PubMed] [Google Scholar]
- [45].Chung WS, Welsh CA, Barres BA, et al. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci 2015;18:1539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cheng Q, Danao J, Talreja S, et al. TREM2-activating antibodies abrogate the negative pleiotropic effects of the Alzheimer's disease variant Trem2(R47H) on murine myeloid cell function. J Biol Chem 2018;293:12620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lessard CB, Malnik SL, Zhou Y, et al. High-affinity interactions and signal transduction between Abeta oligomers and TREM2. EMBO Mol Med 2018;10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cheng-Hathaway PJ, Reed-Geaghan EG, Jay TR, et al. The Trem2 R47H variant confers loss-of-function-like phenotypes in Alzheimer's disease. Mol Neurodegener 2018;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Huang M, Wang D, Xu Z, et al. Lack of genetic association between TREM2 and Alzheimer's disease in East Asian population: a systematic review and meta-analysis. Am J Alzheimers Dis Other Demen 2015;30:541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhou SL, Tan CC, Hou XH, et al. TREM2 variants and neurodegenerative diseases: a systematic review and meta-analysis. J Alzheimers Dis 2019;68:1171–84. [DOI] [PubMed] [Google Scholar]
- [51].Liu G, Liu Y, Jiang Q, et al. Convergent genetic and expression datasets highlight TREM2 in Parkinson's disease susceptibility. Mol Neurobiol 2016;53:4931–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


