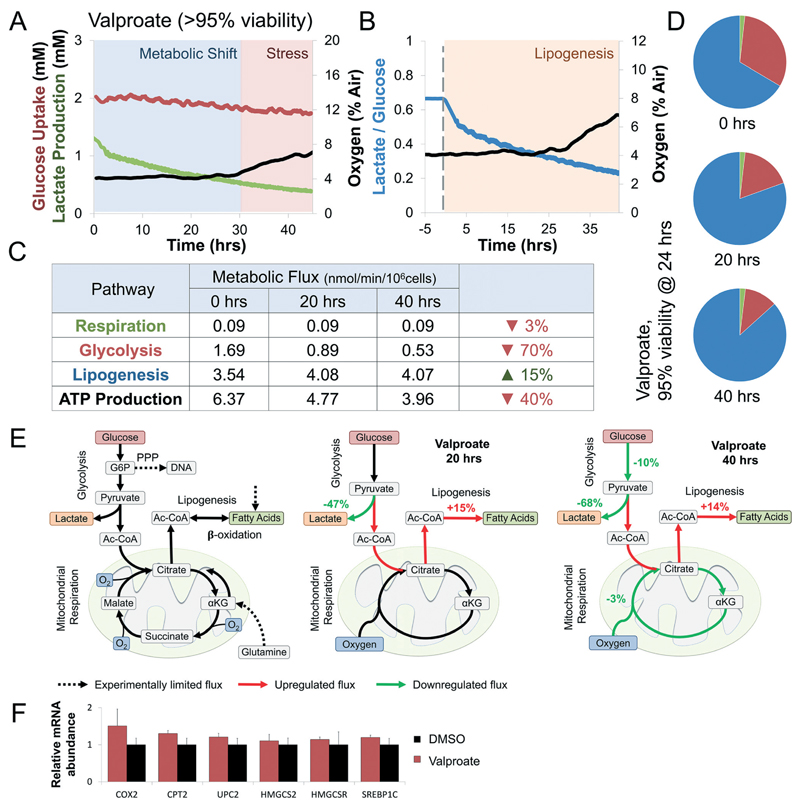
Fig. 4. Valproate induces a rapid shift from glycolysis to lipogenesis at sub-toxic concentrations.
(A) Curves of oxygen, glucose, and lactate fluxes during continuous perfusion with 5 mM Valproate. Oxygen uptake (black) drops by 3% only after 29 hours of continuous exposure. In contrast, glucose uptake (red) and lactate production (green) drop immediately after exposure. Determination of metabolic shift and stress phases are interpretation of the experimental results against trends measured simultaneously of a control bioreactor (Fig. S3A†). Metabolic shift was determined at the onset of a significant change in glucose uptake or lactate production, while stress was determined at the onset of a significant change in oxygen uptake. (B) Changes in lactate over glucose ratio following exposure to Valproate (blue line). Ratio drops by 25% immediately upon exposure, suggesting enzymatic rather than transcriptional effect. Lipogenesis was determined at the onset of a significant shift in the metabolic fluxes towered lipid synthesis (methods) (C) intracellular metabolic fluxes calculated following 0, 20, and 40 hours exposure to sub-toxic concentration Valproate (>95% viability). Glucose utilization in each pathway is shown as nmol min−1 per 106 cells as well as calculated ATP production (methods). Lipogenesis increases by 15% while glycolysis and ATP production drop by 40% and 70%, respectively. (D) Relative glucose utilization is shown as pie chart. Lipogenesis utilizes an increasing percentage of available glucose during Valproate exposure. (E) Schematics depicting the metabolic response of liver cells to Valproate. Dotted arrows note experimentally-limited fluxes, red and green arrows note up- and down-regulated fluxes, respectively. Valproate exposure shift glucose from lactate to citrate production, increasing lipogenesis through the first 30 hours of exposure. Continued exposure suppressed glucose uptake and oxidative respiration, hallmarks of metabolic stress. (F) Gene expression analysis in E6/E7LOW hepatocytes shows metabolic changes but no significant evidence of β-oxidation suppression, supporting enzymatic driven mechanism in sub-toxic exposure.