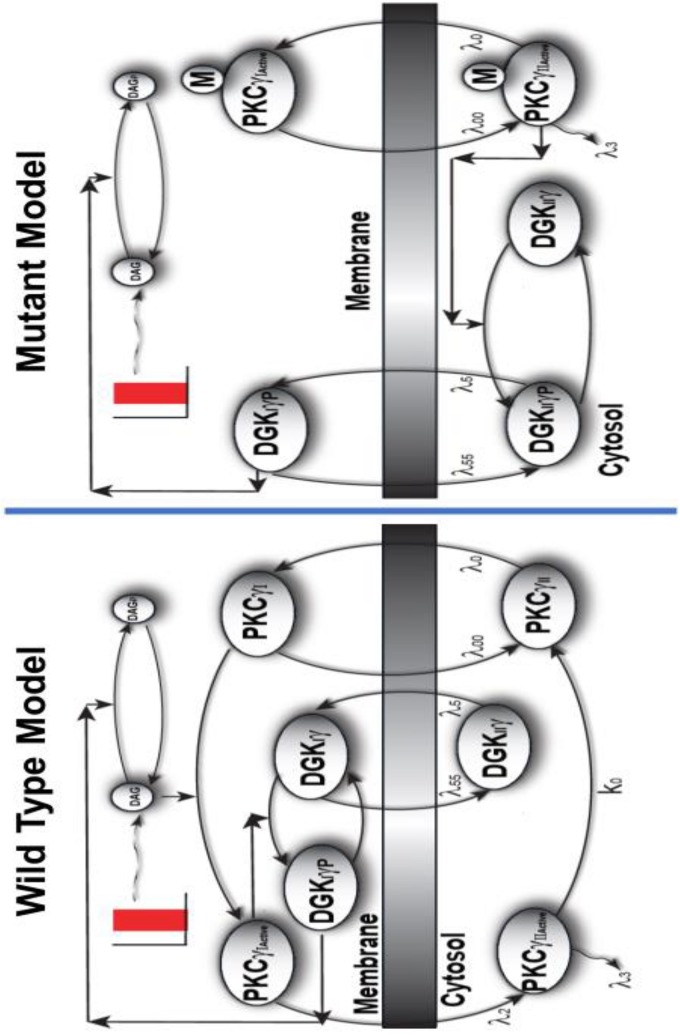
FIGURE 2.
The comparison of two-compartment, wild-type, and mutant models of local DAG signaling in PCs. Depolarization-induced local generation of DAG in PCs, in turn, leads to the functional coupling between PKCγ and DGKγ molecules. The wild-type model is characterized by dormant and inactive PKCγ molecule residing in cytosol, whereas the mutant model is characterized by active PKCγ isoform in the cytosol. In the wild-type model, the depolarization-induced activation of purinergic receptor and local generation of DAG stimulates the translocation of PKCγ and DGKγ molecules from cytosol to membrane compartments. Once in the membrane compartment, the DAG binds with PKCγ and activates it, which, in turn, activates DGKγ through phosphorylation. In the membrane compartment, the phosphorylated and active DGKγ molecule stimulates the DAG metabolism, thus restricting its own activation. Once DAG is converted to PA in membrane compartment, both these molecules return to their dormant forms in cytosol. In contrast, the signaling in the mutant model is reduced as in the mutant model of PCs; the PKCγ is in constitutively active state and leads to the phosphorylation and activation of DGKγ in the cytosol even during unstimulated conditions. On stimulation, as DAG is generated in the membrane compartment, both these molecules migrate to membrane where the already activated DGKγ molecule converts DAG to PA. It seems that signaling in the mutant model is reduced due to the constitutively active form of PKCγ in cytosol.