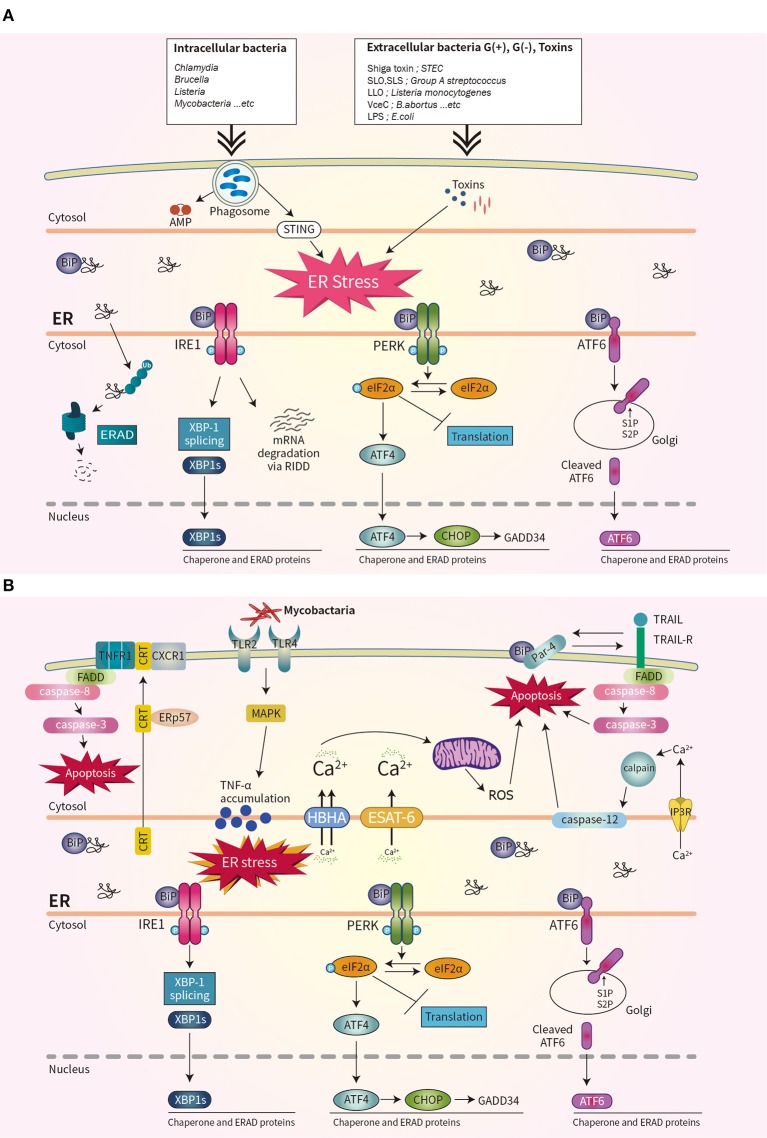
Figure 1.
Schematic overview of unfolded protein response (UPR) signaling during bacterial infection. (A) Three ER stress sensors–IRE1, PERK, and ATF6–are activated when the accumulation of misfolded protein aggregates promotes recruitment of BiP. Bacterial infection and toxins activate the UPR. (B) During mycobacterial infection, co-translocated calreticulin and ERp57 form a complex with TNFR1 and CXCR1 in the plasma membrane, leading to apoptosis, and suppression of intracellular Mtb. The interaction of Par-4 and BiP leads to apoptosis by inducing Mtb-mediated ER stress and activating the FADD/caspase-8/-3 pathway. The mycobacterial antigens HBHA and ESAT-6 affect the ER membrane and induce the release of Ca2+ from the ER to mitochondria, leading to the production of reactive oxygen species and apoptosis.