Fig. 3.
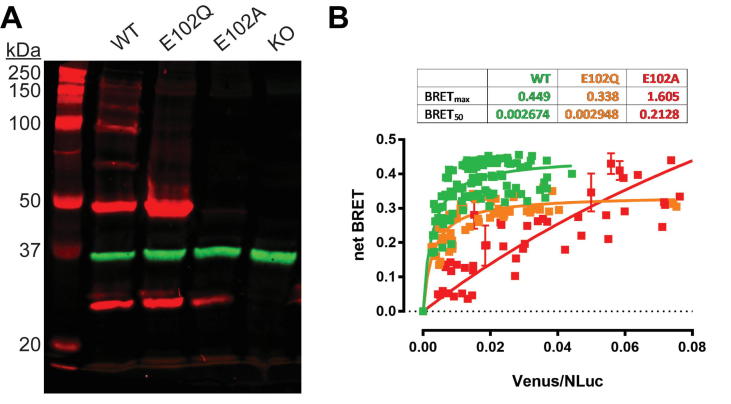
WT σ1R forms high-order oligomers while E102Q and E102A do not. (A) Representative western blot from five experiments are shown to visualize σ1R (red) and GAPDH (green) bands for wildtype (WT), E102Q, and E102A constructs expressed in Δσ1R HEK293T cells. Protein extract from Δσ1R cells without σ1R rescue expression is run in the last lane. (B) Molecular interactions between σ1R monomers are measured by BRET. Δσ1R HEK 293T cells were transfected with a constant amount of the RLuc-fusion construct and increasing amounts of the Venus-fusion construct (green-WT, orange-E102Q, and red-E102A). All data points were performed in triplicate (S.E.M. shown as error bars). The BRETmax and BRET50 values were calculated by nonlinear regression using a single-site saturation binding model. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)