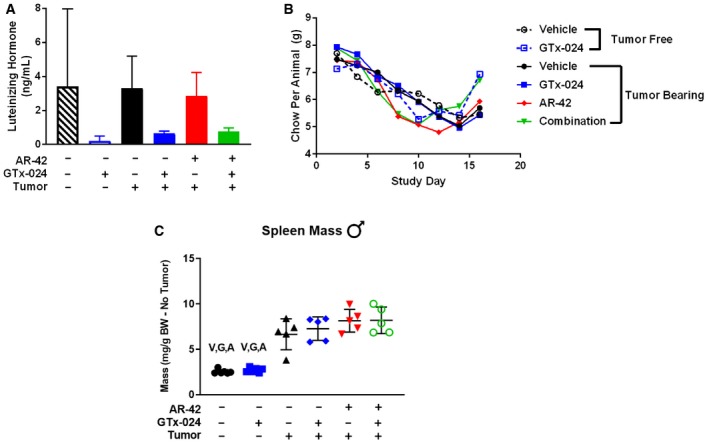
Study 1, tumor‐bearing male mice receiving GTx‐024 (15 mg/kg;
n = 5), AR‐42 (10 mg/kg;
n = 5), combination (15 mg/kg GTx‐024 and 10 mg/kg AR‐42;
n = 5), or vehicle (
n = 5) and tumor‐free mice receiving GTx‐024 (15 mg/kg;
n = 6) or vehicle (
n = 6) were treated daily by oral gavage for 13 days starting 6 days post‐injection of C‐26 cells.
-
A
Serum‐luteinizing hormone levels measured on day 18.
-
B
Per animal food consumption measured every 2 days.
-
C
Spleen weights normalized to tumor mass‐corrected terminal body weight.
Data information: V, G, A indicate statically significant differences versus tumor‐bearing vehicle‐treated, tumor‐bearing GTx‐024‐treated, and tumor‐bearing AR‐42‐treated groups, respectively.
P‐values are provided in
Appendix Table S10, one‐way ANOVA followed by Tukey's multiple comparison test. Means ± SD, except for (B) in which error bars are not shown to improve clarity of presentation. BW, body weight.