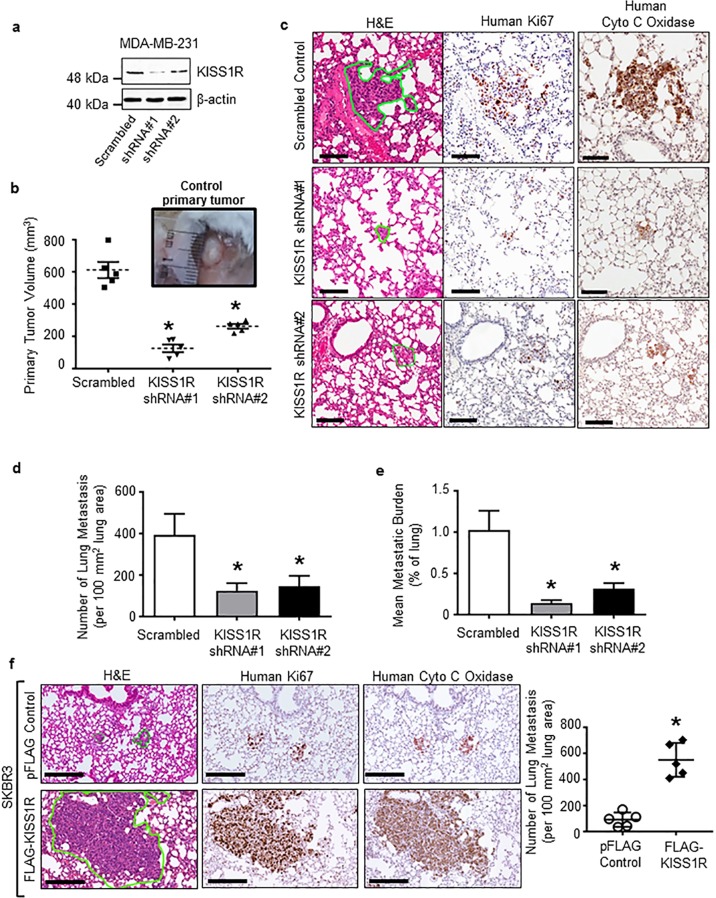
Fig. 2. KISS1R expression regulates primary breast tumor growth and metastasis in xenograft models.
a–e Depletion of endogenous KISS1R in human metastatic triple-negative MDA-MB-231 breast cancer cells inhibits primary tumor growth and lung metastases in a spontaneous metastasis xenograft model. a Representative western blot showing KISS1R expression in lysates from MDA-MB-231 cells stably expressing KISS1R shRNA and scrambled control used in xenograft experiments; β-actin, loading control. Densitometric analysis of blots shown in Supplementary Fig. 1b. b Primary orthotopic tumor volumes: mouse mammary fat pad, 106 cells/mouse, n = 5 mice/group. Points represent each mouse mean tumor volume and bars represent mean volume ± SEM. c Representative images of lung metastasis (outlined in green, left panels) subjected to either hematoxylin and eosin, antihuman Ki67 (middle panels), or human anti-cytochrome C oxidase. Scale bar, 100 μm. The extent of lung metastasis measured by (d) number of lung metastases (normalized to 100 mm2) lung and (e) tumor burden in the lung (percentage of lung occupied by tumor); four sections/tissue/mouse, n = 5 mice per group). Bars represent lung area and number of metastases ± SEM, respectively. One-way ANOVA followed by Dunnett’s multiple comparison test: *p < 0.05. f KISS1R overexpression promotes lung colonization in a experimental metastasis xenograft model. Lung metastases (n = 5 mice/group, outlined in green) formed by human ERα-negative SKBR3 breast cancer cells stably expressing FLAG-KISS1R or pFLAG vector control. Bars represent mean ± SEM. Metastases quantified blindly in hematoxylin and eosin and human anti-cytochrome C oxidase stained slides. Student’s unpaired t test: *p < 0.05. Scale bars, 200 μm.