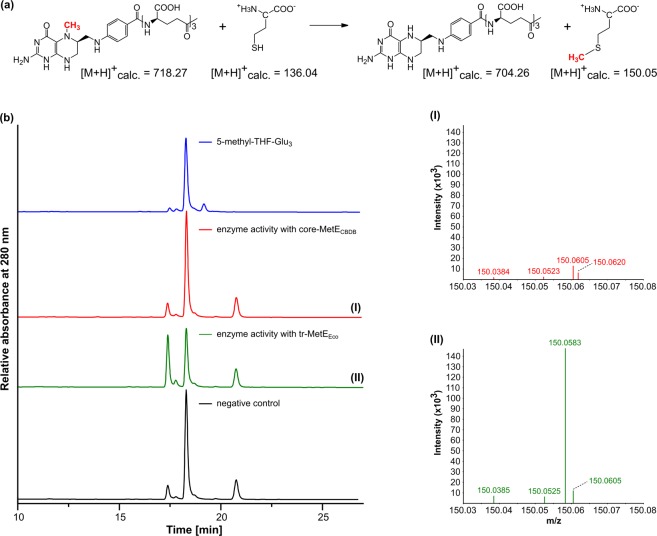
Figure 4.
Core-MetECBDB from D. mccartyi strain CBDB1 does not catalyze the formation of L-methionine with 5-methyl-THF-Glu3 as the methyl donor. (a) Reaction described for tr-MetE from E. coli (tr-MetEEco) catalyzing the methylation of L-homocysteine with 5-methyl-THF-Glu3 to form L-methionine and THF-Glu380. (b) Representative HPLC chromatograms of a chemical standard of 5-methyl-THF-Glu3 (blue), of reaction products after an enzyme activity assay containing 5-methyl-THF-Glu3, D,L-homocysteine and either core-MetECBDB (red) or tr-MetEEco (green) or no enzyme (black). The peak at RT = 21 min represents dithiothreitol added to all reactions. In the presence of tr-MetEEco, 5-methyl-THF-Glu3 (RT = 18 min) reacts to form THF-Glu3 (RT = 17.4 min). Slow demethylation of 5-methyl-THF-Glu3 to THF-Glu3 did occur in the negative control and also in the presence of core-MetECBDB. To evaluate if this demethylation was linked to L-methionine formation, the products of the activity assays were analyzed by mass spectrometry. I) No L-methionine was formed in the presence of core-MetECBDB; II) The product of tr-MetEEco was identified as L-methionine ([M + H]+ = 150.0583 m/z).