Abstract
Background:
The introduction of antibodies targeting programmed cell death protein 1 (PD-1) and programmed cell death-ligand 1 (PD-L1) into clinical practice has had a revolutionary effect on cancer treatment. However, the incidence and risk of fatal adverse events (FAEs) following PD-1/PD-L1 inhibitor administration are controversial.
Methods:
We performed a systematic search for randomized controlled trials (RCTs) of PD-1/PD-L1 inhibitors (atezolizumab, avelumab, durvalumab, nivolumab, and pembrolizumab) in Embase, PubMed, the Cochrane database, and abstracts presented at American Society of Clinical Oncology and European Society of Medical Oncology from inception to July 2018. FAEs were extracted from each study and pooled to calculate overall incidence and odds ratios (ORs).
Results:
In total, 20 RCTs involving 12,398 patients with solid tumors were included in this study. The overall incidence of FAEs with PD-1/PD-L1 inhibitors was 0.43% [95% confidence interval (CI), 0.25–0.66%]. However, the incidences of FAEs varied significantly by tumor type and median follow-up time. Compared with conventional agents, the application of PD-1/PD-L1 inhibitors significantly reduced the risk of FAEs (OR, 0.56; 95% CI, 0.35–0.89; p = 0.015). Moreover, trial sequential analysis confirmed that our results were solid and reliable; further studies were unlikely to alter this conclusion. FAEs occurred dispersed in major organ systems, with the most common mortalities appearing in the respiratory system (46.2%).
Conclusions:
Compared with conventional treatment, PD-1/PD-L1 blockade monotherapy is associated with a significantly reduced risk of mortality in patients with solid tumors.
Keywords: anti-PD-1, anti-PD-L1, fatal adverse event, immunotherapy, meta-analysis
Introduction
Fatal adverse event (FAE) refers to death presumed to have been caused by a drug. FAE is a major cause of fatality globally; previous studies demonstrate that the incidence of fatal adverse drug reactions account for approximately 5% of all deaths in hospital patients,1 and 3% in the general population.2 In cancer patients, the risk of treatment-related mortalities may be even higher because of the serious toxicities induced by conventional treatments.3,4
The programmed cell death protein 1 (PD-1) and programmed cell death-ligand 1 (PD-L1) axis is an important immune checkpoint signaling pathway that downregulates the magnitude of the inflammation response and sustains immune homeostasis.5 In 2014, the United States (US) Food and Drug Administration (FDA) approved pembrolizumab, the first PD-1/PD-L1 inhibitor, for the treatment of melanoma. Since then, agents targeting PD-1/PD-L1 have revolutionized clinical practice in a broad range of tumor types.6,7 Meanwhile, activation of the immune system can also affect healthy tissues and cause a variety of immune-related adverse events (AEs). Previous work has revealed that ipilimumab, an immune checkpoint inhibitor (ICI) targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), could increase the risk of mortality in cancer patients by 130%.8 Although antibodies targeting PD-1/PD-L1 are relatively safe anti-tumor agents, serious adverse events (SAEs) and FAEs have been reported in clinical practice,9–16 and have unfortunate consequences for patients. One previous meta-analysis reported that PD-1/PD-L1 inhibitors have a similar risk of treatment-related death as chemotherapy agents17; evidence was limited because only eight studies were available at that time. Additionally, three of the eight trials were not included in the final analysis since no FAEs were reported.17 An improved understanding of this issue may have important public health and clinical implications given the expected significant increase in the future application of immunotherapy.18 Here, with accumulating evidence, to evaluate the association between PD-1/PD-L1 blockade monotherapy and risk of FAE, we conducted a meta-analysis of randomized controlled trials among patients with solid tumors. Furthermore, we applied trial sequential analysis (TSA) to investigate whether the currently available evidence was sufficient and conclusive.
Materials and methods
This study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.19
Literature search and study selection
A systematic search of the Embase, PubMed, and Cochrane databases from inception to July 2018 was conducted, with no language restrictions. Because recent progress with PD-1/PD-L1 inhibitors might be unpublished, additional searches were carried out through the proceedings of two major international congresses (American Society of Clinical Oncology and European Society of Medical Oncology). The search keywords and medical subject headings used were: atezolizumab, avelumab, durvalumab, nivolumab, pembrolizumab, checkpoint inhibitors, PD-1, PD-L1, and randomized controlled trial (Supplemental Material). All the authors performed the initial search independently, screened titles and abstracts for relevance, and identified trials as excluded, included, or uncertain. For uncertain trials, the full texts were reviewed for confirmation of eligibility. Any discrepancy was resolved by discussion.
Both inclusion and exclusion criteria were prespecified. To be eligible, studies had to meet the following criteria: (1) population: prospective phase II or phase III RCT involving adult patients (>18 years old) with solid tumor; (2) intervention: random assignment of patients to PD-1/PD-L1 inhibitors (atezolizumab, avelumab, durvalumab, pembrolizumab, and nivolumab) monotherapy or control treatment irrespective of dosage and duration; (3) outcomes: available information on FAEs and sample size. Studies were excluded if they were retrospective or prospective observational cohort trials. In addition, Phase I and nonrandomized phase II trials were excluded. Other publications, including review articles, preclinical papers, editorials, and cost-effectiveness analyses, were also excluded. When multiple publications of the same trial occurred, only the most recent or most complete reporting study was included. Any discrepancies were settled by discussion and consensus. All included trials represented unique studies.
Data extraction and risk-of-bias assessment
The key aim of this study was to establish the association between PD-1/PD-L1 inhibitors and FAEs in cancer patients. Eligible abstracts were collected and full texts were reviewed for trial design and reporting of FAEs. The following data were extracted: name of study, trial design, tumor type, number of patients enrolled, number of patients for safety analysis, therapy strategy, median treatment duration, median follow up, median overall survival, and number of FAEs.
The Cochrane risk-of-bias tool was applied to evaluate the risk of bias.20 Every trial was scored as having high, low, or unclear risk of bias to the following criteria: random sequence generation; allocation concealment; blinding of participants and personnel to the study protocol; blinding of outcome assessment; incomplete outcome data and selective reporting. All authors carried out the data extraction and quality assessment independently. Any disagreements were resolved by discussion and consensus.
Trial sequential analysis
Random errors increase the risk of type I error (false-positive results) in meta-analysis because of sparse data or repetitive examining.21 Accordingly, trial sequential analysis (TSA) was introduced.21,22 TSA can determine whether the data in a meta-analysis is reliable and conclusive. When the cumulative z curve crosses the trial sequential monitoring boundary, or enters the futility area, a sufficient level of evidence for the anticipated intervention effect may have been reached and no further trials need be included. If the z curve crosses none of the boundaries, and the required information size has not been reached, there is insufficient evidence to reach a conclusion. Here, we estimated the required information size using α = 0.05 (two-sided), β = 0.20 (power of 80%), the control event proportions calculated for the traditional palpation group, and an odds ratio (OR) reduction of 50% in the first-attempt failure. TSA was conducted using TSA version 0.9.5.9 Beta (http://www.ctu.dk/tsa).
Statistical analysis
The primary analysis investigated the overall incidence, OR, and corresponding 95% confidence interval (CI) of FAEs in patients treated with PD-1/PD-L1 inhibitors. To calculate the incidence, the number of patients and the number of FAEs were extracted from every trial. For calculation of OR, patients assigned to PD-1/PD-L1 inhibitor arm were compared with those assigned to a control arm. When trials reported no FAE in one arm, we applied a classic half-integer continuity correction to calculate OR.
Statistical heterogeneity across trials was evaluated by Cochrane’s Q statistic. The I2 statistic was calculated to assess the extent of inconsistency contributable to the heterogeneity across different studies.23 The assumption of homogeneity was considered invalid for I2 > 25% and p < 0.05. Summary ORs and incidences were calculated using fixed-effects or random-effects models depending on the heterogeneity. To check the impact of various clinicopathological variables on FAE, we further conducted post hoc subgroup analysis based on tumor type, PD-1/PD-L1 inhibitors, clinical phase, control type, masking method, year of publication, and median follow up.
Potential publication bias was assessed by visual inspection of a funnel plot, and also evaluated using the tests of Egger and colleagues and Begg and colleagues.24,25 Two-sided p < 0.05 were considered statistically significant. All analysis was performed by MedCalc 18.2.1 (MedCalc Software, Belgium).
Results
Search results
In total, 8397 potentially related papers were identified from the initial search, including 3815 from PubMed, 4109 from EMBASE, and 473 from the Cochrane database. Of this total, 3694 articles were removed because of duplications. After screening of titles and abstracts, 4597 studies did not meet the inclusion criteria. Following further review of the whole texts of the remaining 106 potentially eligible articles, 20 RCTs were enrolled for the final analysis.9–15,26–39 A flow chart showing study selection is presented in Figure 1.
Figure 1.
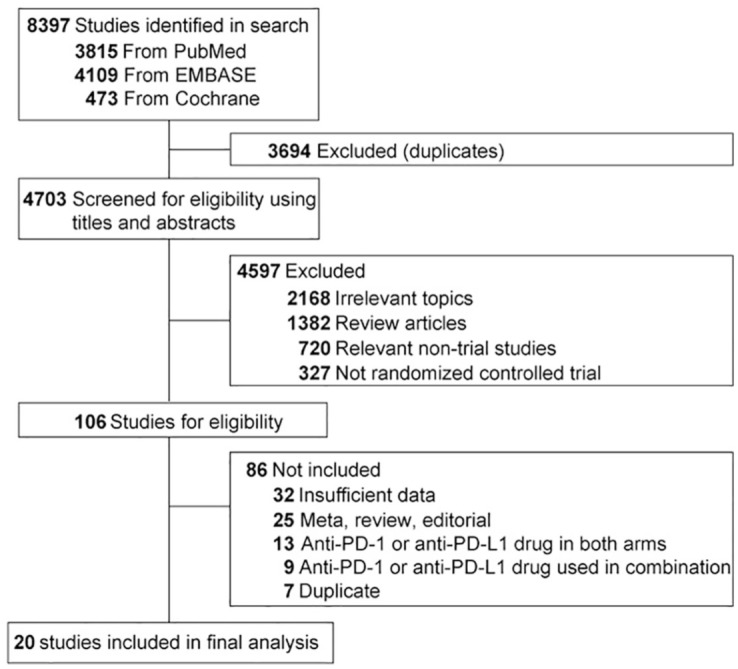
Selection of trials included in this study.
Study characteristics
The main characteristics of the 20 eligible RCTs are presented in Table 1. All these studies were international, multicenter, RCTs funded by the pharmaceutical industry. Randomized treatments were well generated in all trials. Of the 20 eligible RCTs, 14 were open-labeled,9–11,13,14,29–33,35–37,39 6 were double-blinded,12,15,26,27,34,38 and thus treated as being of low risk of performance and detection bias. Follow-up time was adequate for each trial. The methodological qualities of the included trials were generally moderate to good (Supplemental Table S1).
Table 1.
Characteristics of the trials included in this study.
| Study | Trial design | Tumor type | No. of patients (enrolled) | Median age (years, range) | Sex (M/F) | No. of patients (safety) | Treatment | Median treatment time (months, range) | Median follow up (months, range) | Median OS (months, 95% CI) | FAE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CheckMate 0579 | Open-label, phase III RCT | Lung cancer | 292 | 61(37–84) | 151/141 | 287 | Nivolumab 3 mg/kg every 14 days | 3.0(0.5–26.0) | >13.2 | 12.2(9.7–15.0) | 1 |
| 290 | 64(21–85) | 168/122 | 268 | Docetaxel 75 mg/m2 every 21 days | 3.0(0.8–17.3) | 9.4(8.1–10.7) | 1 | ||||
| CheckMate 01710 | Open-label, phase III RCT | Lung cancer | 135 | 62(39–85) | 111/24 | 131 | Nivolumab 3 mg/kg every 14 days | 4.0(0.5–24.0) | <11.0 | 9.2(7.3–13.3) | 0 |
| 137 | 64(42–84) | 97/40 | 129 | Docetaxel 75 mg/m2 every 21 days | 2.3(0.8–21.8) | 6.0(5.1–7.3) | 3 | ||||
| CheckMate 02611 | Open-label, phase III RCT | Lung cancer | 271 | 63(32–89) | 184/87 | 267 | Nivolumab 3 mg/kg every 14 days | 3.7(0.0–26.9) | 13.5 | 14.4(11.7–17.4) | 2 |
| 270 | 65(29–87) | 148/122 | 263 | ICC once every 21 days | 3.4(0.0–20.9) | 13.2(10.7–17.1) | 3 | ||||
| CheckMate 14113 | Open-label, phase III RCT | Head and neck cancer | 240 | 59(29–83) | 197/43 | 236 | Nivolumab 3 mg/kg every 14 days | 1.9 | 5.1(0–16.8) | 7.5(5.5–9.1) | 2 |
| 121 | 61(28–78) | 103/18 | 111 | Standard therapy | 1.9 | 5.1(4.0–6.0) | 1 | ||||
| ATTRACTION-212 | Double-blind, phase III RCT | G/GJC | 330 | 62(54–69) | 229/101 | 330 | Nivolumab 3 mg/kg every 14 days | 1.9 | 8.9 | 5.3(4.6–6.4) | 5 |
| 163 | 61(53–68) | 119/44 | 161 | Placebo 3 mg/kg every 14 days | 1.9 | 8.6 | 4.1(3.4–4.9) | 2 | |||
| CheckMate 02514 | Open-label, phase III RCT | Renal cancer | 410 | 62(23–88) | 315/95 | 406 | Nivolumab 3 mg/kg every 14 days | 5.5(0–29.6) | >14.0 | 25.0(21.8–NR) | 0 |
| 411 | 62(18–86) | 304/107 | 397 | Everolimus 10 mg daily | 3.7(0.2–25.7) | 19.6(17.6–23.1) | 2 | ||||
| CheckMate 06615 | Double-blind, phase III RCT | Melanoma | 210 | 64(18–86) | 121/89 | 206 | Nivolumab 3 mg/kg every 14 days | NR | <16.7 | Not reached | 0 |
| 205 | 66(26–87) | 125/83 | 205 | Docetaxel 1000 mg/m2 every 21 days | 10.8(9.3–12.1) | 0 | |||||
| CheckMate 23826 | Double-blind, phase III RCT | Melanoma | 453 | 56(19–83) | 258/195 | 452 | Nivolumab 3 mg/kg every 14 days | 12.0 | >18.0 | NR | 0 |
| 453 | 54(18–86) | 269/184 | 453 | Ipilimumab 10 mg/kg every 21 days | 3.0 | 2 | |||||
| CheckMate 06727 | Double-blind, phase III RCT | Melanoma | 316 | 60(25–90) | 202/114 | 313 | Nivolumab 3 mg/kg every 14 days | 7.5 | 35.7 | 37.6(29.1–NR) | 1 |
| 315 | 62(18–89) | 202/113 | 311 | Ipilimumab 3 mg/kg every 21 days | 3.0 | 18.6 | 19.9(16.9–24.6) | 1 | |||
| CheckMate 03728,29 | Open-label, phase III RCT | Melanoma | 272 | 59(23–88) | 176/96 | 268 | Nivolumab 3 mg/kg every 14 days | 5.3 | 8.4 | 15.7(12.9–19.9) | 0 |
| 133 | 62(29–85) | 85/48 | 102 | ICC | 2.0 | 14.4(11.7–18.2) | 0 | ||||
| POPLAR30 | Open-label, phase II RCT | Lung cancer | 144 | 62(42–82) | 93/51 | 142 | Atezolizumab 1200 mg every 21 days | 3.7(0–19) | 14.8(0.2–19.6) | 12.6(9.7–16.4) | 1 |
| 143 | 62(36–84) | 76/67 | 135 | Docetaxel 75 mg/m2 every 21 days | 2.1(0–17) | 15.7(0.1–18.7) | 9.7(8.6–12.0) | 3 | |||
| IMvigor21131 | Open-label, phase III RCT | Urothelial cancer | 467 | 67(33–88) | 357/100 | 459 | Atezolizumab 1200 mg every 21 days | 2.8(0–24.0) | 17.3(0–24.5) | 11.1(8.6–15.5) | 4 |
| 464 | 67(31/84) | 361/103 | 443 | ICC once every 21 days | 2.1(0–23.0) | 10.6(8.4–12.2) | 9 | ||||
| OAK32 | Open-label, phase III RCT | Lung cancer | 425 | 63(33–82) | 261/164 | 609 | Atezolizumab 1200 mg every 21 days | 3.4(0–26.0) | 21 | 13.8(11.8–15.7) | 0 |
| 425 | 64(34–85) | 259/166 | 578 | Docetaxel 75 mg/m2 every 21 days | 2.1(0–23.0) | 9.6(8.6–11.2) | 1 | ||||
| KEYNOTE-04533 | Open-label, phase III RCT | Urothelial cancer | 270 | 67(29–88) | 200/70 | 266 | Pembrolizumab 200 mg every 21 days | 3.5(0–20.0) | 14.1(9.9–22.1) | 10.3(8.0–11.8) | 1 |
| 272 | 65(26–84) | 202/70 | 255 | ICC once every 21 days | 1.5(0–14.2) | 7.4(6.1–8.3) | 4 | ||||
| KEYNOTE-05434 | Double-blind, phase III RCT | Melanoma | 514 | 54(19–88) | 324/190 | 509 | Pembrolizumab 200 mg every 21 days | 13.5 | 14.7 | NR | 1 |
| 505 | 54(19–83) | 304/201 | 502 | Placebo 200 mg every 21 days | 13.5 | 15.4 | 0 | ||||
| KEYNOTE-01035 | Open-label, phase II/III RCT | Lung cancer | 344 | 63(56–69) | 212/132 | 339 | Pembrolizumab 2 mg/kg every 21 days | 3.5 | 13.1 | 10.4(9.4–11.9) | 3 |
| 346 | 63(56–69) | 213/133 | 343 | Pembrolizumab 10 mg/kg every 21 days | 3.5 | 12.7(10.0–17.3) | 3 | ||||
| 343 | 62(56–69) | 209/134 | 309 | Docetaxel 75 mg/m2 every 21 days | 2.0 | 8.5(7.5–9.8) | 5 | ||||
| KEYNOTE-06136 | Open-label, phase III RCT | G/GJC | 296 | 63(54–70) | 202/94 | 294 | Pembrolizumab 200 mg every 21 days | 4.4 | 7.9 | 9.1(6.2–10.7) | 3 |
| 296 | 60(53–68) | 208/88 | 276 | Paclitaxel 80 mg/m2 on day 1,8,15 every 28 days | 3.5 | 8.3(7.6–9.0) | 1 | ||||
| KEYNOTE-02437 | Open-label, phase III RCT | Lung cancer | 154 | 65(33–90) | 92/62 | 154 | Pembrolizumab 200 mg every 21 days | 7.0(0–18.7) | 11.2(6.3–19.7) | Not reached | 1 |
| 151 | 66(38–85) | 95/56 | 150 | Placebo 200 mg every 21 days | 3.5(0–16.8) | 3 | |||||
| KEYNOTE-00238 | Double-blind, phase II RCT | Melanoma | 180 | 62(15–87) | 104/76 | 178 | Pembrolizumab 2 mg/kg every 21 days | 3.8(0–16.6) | 10.0 | NR | 0 |
| 181 | 60(27–89) | 109/72 | 179 | Pembrolizumab 10 mg/kg every 21 days | 4.8(0–16.8) | 0 | |||||
| 179 | 63(27–87) | 114/165 | 171 | ICC | 2.0(0–11.2) | 0 | |||||
| KEYNOTE-00639 | Open-label, phase III RCT | Melanoma | 279 | 61(18–89) | 161/118 | 278 | Pembrolizumab 10 mg/kg every 14 days | 7.0(0–27.0) | 22.9 | Not reached | 1 |
| 277 | 63(22–89) | 174/103 | 277 | Pembrolizumab 10 mg/kg every 21 days | 6.0(0–27.8) | Not reached | 0 | ||||
| 278 | 62(18–88) | 162/116 | 256 | Ipilimumab 3 mg/kg every 21 days | 2.3(0–3.3) | 16.0(13.5–22.0) | 0 |
FAE, fatal adverse event; G/GJC, gastric or gastroesophageal junction cancer; ICC, investigator-choice chemotherapy; NR, not reported; OS, overall survival; RCT, randomized controlled trial.
A total of 12,398 patients were included for safety analysis; 6923 (56%) subjects were treated by PD-1/PD-L1 inhibitors and 5475 subjects (44%) were enrolled in control arms. The average numbers of patients recruited in these eligible trials was 620 (range, 260–1187). Of the 20 studies, 17 were phase III RCTs, POPLAR and KEYNOTE-002 were phase II RCTs,30,38 and KEYNOTE-010 was a phase II/III trial.35 Nivolumab was examined in 10 trials with 5296 patients, pembrolizumab in 7 trials with 4736 patients, and atezolizumab in 3 trials with 2366 patients. Underlying malignancies included lung cancer (seven studies, 4104 patients), melanoma (seven trials, 4660 patients), urothelial cancer (two trials, 1423 patients), gastric or gastroesophageal junction cancer (G/GJC, two trials, 1061 patients), renal cancer (one trial, 803 patients), and head and neck cancer (one trial, 347 patients). A total of 14 RCTs used chemotherapy as a control; other controls included Everolimus,14 placebo,12,34,37 and ipilimumab.26,27,39
FAEs and PD-1/PD-L1 inhibitors
In total, there were 70 FAEs (PD-1/PD-L1 inhibitors, 29; control, 41) in the eligible trials. The highest incidence of FAE associated with PD-1/PD-L1 inhibitors was observed in G/GJC (1.42%; 95% CI, 0.65–2.69%), and the lowest incidence was observed in melanoma (0.26%; 95% CI, 0.10–0.54%). Using a random-effects model (heterogeneity test: Q = 32.37; p = 0.03; I2 = 41.3%), the summary incidence of FAEs in patients receiving PD-1/PD-L1 inhibitors was 0.43% (95% CI, 0.25–0.66%). We explored the possible causes of the heterogeneity. As shown in Table 2, the incidence of FAEs varies significantly by tumor type (p = 0.008) and median follow-up time (p = 0.046), suggesting that these factors contribute to the risk of FAEs.
Table 2.
Subgroup analysis of the incidences and ORs of FAEs with PD-1/PD-L1 inhibitors.
| No. of trials | No. of FAEs/no. of patients |
Incidence of FAE, %(95% CI) |
OR (95% CI) | p | |||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||
| Tumor type | |||||||
| Lung cancer | 7 | 11/2272 | 19/1832 | 0.50(0.25–0.88) | 1.03(0.62–1.60) | 0.43(0.21–0.90) | 0.02 |
| Melanoma | 7 | 3/2660 | 3/2000 | 0.26(0.10–0.54) | 0.30(0.11–0.66) | 0.76(0.24–2.42) | 0.65 |
| Urothelial cancer | 2 | 5/725 | 13/698 | 0.80(0.29–1.76) | 1.99(1.09–3.32) | 0.37(0.13–1.03) | 0.06 |
| G/GJC | 2 | 8/624 | 3/437 | 1.42(0.65–2.69) | 0.84(0.21–2.21) | 1.67(0.44–6.31) | 0.45 |
| PD-1/PD-L1 inhibitor | |||||||
| Nivolumab | 10 | 11/2896 | 15/2400 | 0.43(0.23–0.74) | 0.79(0.48–1.23) | 0.57(0.27–1.19) | 0.13 |
| Atezolizumab | 3 | 5/1210 | 13/1156 | 0.48(0.01–1.64) | 1.31(0.16–3.56) | 0.39(0.14–1.05) | 0.06 |
| Pembrolizumab | 7 | 13/2817 | 13/1919 | 0.54(0.30–0.89) | 0.73(0.21–1.58) | 0.67(0.32–1.41) | 0.29 |
| Clinical phase | |||||||
| Phase III | 18 | 28/6424 | 38/5169 | 0.46(0.31–0.66) | 0.82(0.49–1.22) | 0.57(0.36–0.92) | 0.02 |
| Phase II | 2 | 1/499 | 3/306 | 0.43(0.06–1.48) | 1.25(0.33–3.23) | 0.34(0.05–2.42) | 0.28 |
| Control type | |||||||
| Chemotherapy | 14 | 21/4358 | 34/3395 | 0.50(0.29–0.85) | 1.14(0.72–1.66) | 0.57(0.37–0.84) | 0.01 |
| Other therapy | 6 | 8/2565 | 7/2080 | 0.32(0.08–0.72) | 0.47(0.23–0.87) | 0.70(0.27–1.82) | 0.46 |
| Masking method | |||||||
| Double blind | 6 | 7/2167 | 5/1803 | 0.40(0.12–0.85) | 0.46(0.21–0.90) | 0.84(0.30–2.35) | 0.73 |
| Open label | 14 | 22/4756 | 36/3672 | 0.50(0.32–0.74) | 1.11(0.70–1.61) | 0.48(0.28–0.81) | 0.01 |
| Year of publication | |||||||
| 2015–2016 | 11 | 11/3478 | 19/2555 | 0.32(0.12–0.61) | 0.83(0.39–1.42) | 0.44(0.20–0.94) | 0.03 |
| 2017–2018 | 9 | 18/3445 | 22/2920 | 0.57(0.28–0.95) | 0.74(0.30–1.36) | 0.70(0.36–1.36) | 0.29 |
| Median follow up | |||||||
| >12 months | 12 | 18/4947 | 31/4170 | 0.37(0.22–0.59) | 0.82(0.46–1.28) | 0.46(0.26–0.82) | 0.01 |
| <12 months | 7 | 11/1770 | 10/1100 | 0.75(0.40–1.27) | 1.17(0.62–1.99) | 0.73(0.32–1.67) | 0.46 |
| Overall | 20 | 29/6923 | 41/5475 | 0.43(0.25–0.66) | 0.78(0.46–1.18) | 0.56(0.35–0.89) | 0.02 |
CI, confidence interval; FAE, fatal adverse event; G/GJC, gastric or gastroesophageal junction cancer; OR, odds ratio; PD-1/PD-L1, programmed cell death protein 1/programmed cell death-ligand 1.
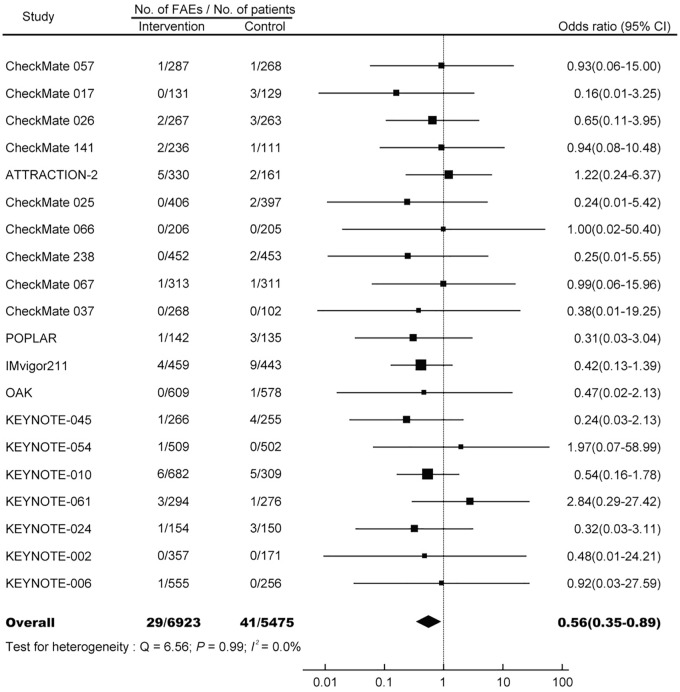
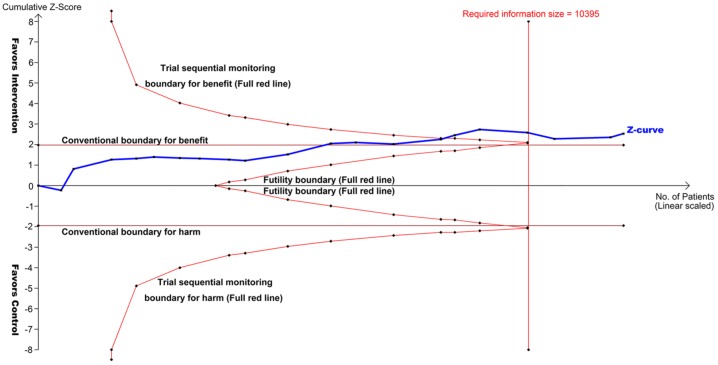
The summary OR of FAE induced by PD-1/PD-L1 monotherapy is 0.56 (95% CI, 0.35–0.89; p = 0.015; Figure 2), suggesting a significantly decreased risk of FAEs associated with PD-1/PD-L1 inhibitors compared with controls. No heterogeneity was identified (Q = 6.56; p = 0.99; I2 = 0.0%). TSA reveals that the cumulative z curve crosses the boundary for required information size (Figure 3), which established sufficient and conclusive evidence. Thus, further trials were not needed and were unlikely to change our results.
Figure 2.
OR of FAEs associated with PD-1/PD-L1 monotherapy versus control.
FAE, fatal adverse event; OR, odds ratio.
Figure 3.
TSA of 20 RCTs comparing PD-1/PD-L1 inhibitors with controls (scaled trial distance). TSA demonstrated that the cumulative z curve crossed the boundary for required information size, establishing conclusive and sufficient evidence, and suggesting no further trials are needed. A diversity-adjusted required information size of 10,395 patients was calculated using α = 0.05 (two-sided) and β = 0.20 (power of 80%), an anticipated relative risk reduction of 50% in the control arm. X-axis, number of patients randomized; Y-axis, cumulative z score; horizontal red lines, conventional boundaries (z score, ±1.96; two-sided p = 0.05); sloping red lines with black dots, trial sequential monitoring boundaries; blue line with black dots, z curve; vertical red line, required information size.
PD-1/PD-L1, programmed cell death protein 1/programmed cell death-ligand 1; RCT, randomized controlled trial; TSA, trial sequential analysis.
Subgroup analyses
To examine whether tumor type influenced the risk of FAEs with PD-1/PD-L1 inhibitors, we conducted subgroup analysis based on tumor type (Table 2). The highest OR was found in G/GJC (OR, 1.67; 95% CI, 0.44–6.31; incidence, 1.42% versus 0.84%), and the lowest OR was observed in urothelial cancer (OR, 0.37; 95% CI, 0.13–1.03 incidence, 0.80% versus 1.99%). The odds ratio of FAEs showed statistical differences by tumor type (p = 0.04), suggesting the risk of FAE associated with PD-1/PD-L1 inhibitors was different among various tumor types.
To investigate whether the association between risk of FAE and PD-1/PD-L1 inhibitors could be altered by other clinicopathological characteristics, we also performed subgroup analysis according to PD-1/PD-L1 inhibitor, clinical phase, control type, masking method, year of publication, and median follow-up time (Table 2). No statistical difference was observed among all these subgroup analyses (p > 0.05 for all analyses).
Specific FAEs
Among the total 29 FAEs associated with PD-1/PD-L1 blockade treatment, 26 (89.7%) had specified adverse events, the rest were deaths (n = 3, 10.3%) due to unknown causes (Table 3). Detailed causes of FAEs are presented in Supplemental Table S2. Respiratory system (pneumonitis, pneumonia, and respiratory failure) had the most frequently occurring FAE, reported in 10 studies and representing a total of 12 deaths (46.2%) from of all specified FAEs. Other common reported FAEs occurred in heart (n = 3, 11.5%), and gastro-intestinal (n = 2, 7.7%) systems.
Table 3.
Incidences and OR of specific FAEs with PD-1/PD-L1 inhibitors.
| FAEs | No. of trials | No. of FAEs/no. of patients |
Incidence of FAE, %(95% CI) |
OR (95% CI) | p | ||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||
| Specified | 20 | 26/6923 | 35/5475 | 0.43(0.29–0.61) | 0.75(0.54–1.01) | 0.56(0.35–0.91) | 0.02 |
| Unspecified | 6 | 3/1645 | 6/1420 | 0.25(0.07–0.63) | 0.54(0.23–1.08) | 0.54(0.17–1.66) | 0.28 |
| Respiratory system | 10 | 12/3416 | 9/2660 | 0.41(0.22–0.68) | 0.39(0.19–0.71) | 0.88(0.41–1.89) | 0.74 |
| Blood | 7 | 1/2128 | 6/1706 | 0.11(0.02–0.37) | 0.48(0.21–0.93) | 0.42(0.14–1.27) | 0.12 |
| Gastrointestine | 5 | 2/1848 | 3/1644 | 0.17(0.03–0.48) | 0.24(0.07–0.62) | 0.70(0.21–2.41) | 0.58 |
| Cardiac | 4 | 3/1613 | 2/1048 | 0.24(0.06–0.62) | 0.31(0.07–0.87) | 0.87(0.21–3.58) | 0.85 |
| Overall | 20 | 29/6923 | 41/5475 | 0.43(0.25–0.66) | 0.78(0.46–1.18) | 0.56(0.35–0.89) | 0.02 |
CI, confidence interval; FAE, fatal adverse event; OR, odds ratio; PD-1/PD-L1, programmed cell death protein 1/programmed cell death-ligand 1.
Publication bias
Inspection of the funnel plot, and formal statistical tests (Begg’s test, p = 0.65; Egger’s test, p = 0.58), reveals no evidence of publication bias (Supplemental Figure S1).
Discussion
Here, based on 20 RCTs with 12,398 patients, our results revealed that the overall incidence of mortalities induced by PD-1/PD-L1 monotherapy was 0.43%. Compared with conventional treatment, PD-1/PD-L1 blockade immunotherapy decreased the risk of FAEs by 44%. TSA confirmed that our results were solid and reliable, further studies were unlikely to alter our findings. In addition, FAEs occurred in major organ systems in a dispersed manner, with the most common toxicities appearing in the respiratory system. These findings suggest that PD-1/PD-L1 blockade therapy is a relatively safe treatment regimen compared with conventional interventions. Although uncommon, fatal toxic effects occurred at a rate of 0.43% in PD-1/PD-L1 monotherapy. Clinicians should be aware of potential lethal complications. Moreover, future studies are needed to identify patients at high risk of FAE to establish optimal monitoring strategies and explore new methods to reduce risk.
Meta-analysis is a powerful tool for investigating rare events like FAE, given that it can comprehensively synthesize data from various trials to achieve a robust and reliable result. One previous study showed that there was no association between PD-1/PD-L1 inhibitors and risk of FAEs in cancer patients.17 In this meta-analysis, eight trials with 4292 patients (experiment, 2498; control, 1794) were included in the PD-1/PD-L1 subgroup analysis. The incidence of FAEs was 0.52%, close to our estimate of 0.43% in the present study. However, the OR of FAEs was 0.63 (95% CI, 0.31–1.30), which was not consist with the primary result of our study. It should be noted that there were several differences between these two studies. First, our study included 20 well-conducted, high-quality RCTs, with over 12,000 adult patients with solid tumors. Moreover, the lack of heterogeneity in the risk of FAEs suggested good selection of eligible RCTs. With the increased statistical power of more than 8000 cases, our meta-analysis is the most up-to-date and comprehensive study to date, and should be more reliable and solid. Second, to further increase the robustness of our result, we conducted TSA to access the impact of potential random errors and repetitive testing. The analysis revealed that our study established sufficient and conclusive evidence. Third, we investigated the relationships between various clinicopathological characteristics and PD-1/PD-L1 monotherapy-related FAEs.
The development of immunotherapy has greatly improved clinical outcomes in various types of cancers, and immunotherapy is now increasingly used in earlier disease settings and in combination with other therapies.5,40 However, high-grade AEs, SAEs, even FAEs, can occur during treatment. There have been several meta-analyses estimating FAEs associated with ICIs.8,17 Currently, it is generally accepted that the use of ipilimumab is associated with an increased risk of FAEs compared with controls.8 Interestingly, the observed immune-associated toxicities in patients receiving PD-1/PD-L1 inhibitors are similar to those related to anti-CTLA-4; however, the frequency is significantly lower in PD-1/PD-L1 blockade therapy. One possible explanation is that the PD-1/PD-L1 checkpoint acts at a later stage of the T cell response, which causes more limited T cell reactivity toward cancer cells, so these PD-1/PD-L1 inhibitors are generally well tolerated in clinical practice. Additionally, it should be noted that most ipilimumab trials were conducted relatively early, when knowledge about immune-associated AEs and management guidelines for such toxicities had not yet been established.
A recent meta-analysis revealed that taking one ICI was generally safer than taking two ICIs, or one ICI combined with conventional therapy.41 It is plausible that, compared with monotherapy, combination therapy is associated with more FAEs. However, no direct or indirect comparison of FAEs between combination immunotherapy and conventional therapy has been conducted currently. This is a potential focus of future study.
Here, we evaluated the relationship of PD-1/PD-L1 blockade therapy with FAEs based on several different clinicopathological characteristics. The incidence of FAEs varied significantly among cancer types, reflecting the potential association of underlying tumor biology and PD-1/PD-L1 blockade therapy. Further analysis revealed that the risk of FAEs was significantly decreased in lung cancer. In contrast, the ORs of FAEs did not show any statistical differences in other types of cancer. This might suggest that better benefit/risk evaluation could be achieved though PD-1/PD-L1 blockade therapy in lung cancer treatment. With accumulating knowledge about side effects related to immunotherapy, it is speculated that the incidence of FAE will decrease with time. Here, we calculated the incidence and risk of FAE associated with PD-1/PD-L1 immunotherapy in studies published before 2017, and after 2017. However, our data failed to support this hypothesis. We think part of reason is that most of these studies were published between 2015 and 2017 (n = 18, 90%). This time period is too short to observe any significant differences. In addition, the timing when SAE/FAE occurred could have had important clinical implications. However, we cannot conduct such a subgroup analysis here because of the limited information provided in the original publications. This is a potential focus for future study.
Previous studies have revealed that ipilimumab treatment-related deaths are dominated by colitis.42,43 However, our study showed that almost 50% of FAEs induced by PD-1/PD-L1 blockade treatment occurred in the respiratory system. Accordingly, doctors should pay attention to any patient presenting with pulmonary symptoms, including dry cough, hypoxia, and shortness of breath. Radiologically, evidence of interstitial pneumonitis could be presented by high-resolution computed tomography.
This study has several important clinical implications. In clinical practice, the careful review of potential treatment-related toxicities should play an essential role in the decision-making process. In addition, the risk of mortality should be acknowledged to patients before they consent to any therapy. However, doctors often overestimate the beneficial side and overlook the harmful side of a treatment.44,45 Our findings here could play an important role in considering the risk/benefit trade-off of cancer treatment by providing data on the overall incidence and relative risk of FAEs in PD-1/PD-L1 blockade therapy.
This study is restricted by some limitations. First, there was significant heterogeneity in the incidence of FAEs among the included trials. We adjusted for this heterogeneity by applying a random-effects model to determine the overall incidence of FAEs. Even so, this might underestimate the real event rate, given that trials without any deaths received disproportional weight in the calculations. Second, these clinical trials were usually not designed specifically to address toxic events; thus, asymptomatic AEs may have been ignored in prospective assessment. Third, some eligible trials were open-label RCTs. Even for the double-blinded studies, skillful doctors might identify AEs induced by PD-1/PD-L1 inhibitors, which could lead to potential bias. Fourth, since the follow-up time for most eligible RCTs was relatively short, long-term observation of potential serious toxic effects is needed. Fifth, the eligible trials were carried out at different medical centers by various researchers, and might have potential bias in reporting FAEs. Especially, the attribution of FAE to PD-1/PD-L1 treatment was determined by the investigators, and thus associated with some subjectivity. The exact reason for mortality may not be fully studied even at single-patient level. Our study is subject to any biases or errors of the original investigators, and the results are generalizable only to the patient groups eligible for these trials.
Conclusion
PD-1/PD-L1 blockade monotherapy, compared with conventional treatment, significantly reduces the risk of FAEs in patients with solid tumors. This suggests that a better benefit/risk evaluation could be achieved in PD-1/PD-L1 immmonotherapy. However, clinicians should be aware of rare, but potentially lethal, complications, and ensure rigorous monitoring to improve outcomes.
Supplemental Material
Supplemental material, suppl_material for Fatal adverse events associated with programmed cell death protein 1 or programmed cell death-ligand 1 monotherapy in cancer by Bin Zhao, Hong Zhao and Jiaxin Zhao in Therapeutic Advances in Medical Oncology
Acknowledgments
Authors Bin Zhao, Hong Zhao, and Jiaxin Zhao contributed equally to this work.
Footnotes
Author Note: Hong Zhao is now affiliated with The Cancer Center of the Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and publication of this article: This work was funded by National Natural Science Foundation of China (grant number 31571417), Postdoctoral Science Foundation of China (grant numbers 2018M641862 and 2019T120282), and Wenzhou Municipal Science and Technology Bureau (grant number Y20180086). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Bin Zhao  https://orcid.org/0000-0002-5990-1773
https://orcid.org/0000-0002-5990-1773
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Bin Zhao, Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University, 109 Xueyuan West Rd, Wenzhou, 325035, China.
Hong Zhao, The Third Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China.
Jiaxin Zhao, Zhuhai People’s Hospital, Zhuhai Hospital affiliated with Jinan University, Zhuhai, China.
References
- 1. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 2. Wester K, Jönsson AK, Spigset O, et al. Incidence of fatal adverse drug reactions: a population based study. Brit J Clin Pharmacol 2008; 65: 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao B, Zhao H, Zhao J. Risk of fatal adverse events in cancer patients treated with sunitinib. Crit Rev Oncol Hematol 2019; 137: 115–122. [DOI] [PubMed] [Google Scholar]
- 4. Zhao H, Guo L, Zhao H, et al. CXCR4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget 2015; 6: 5022–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kythreotou A, Siddique A, Mauri FA, et al. PD-L1. J Clin Pathol 2018; 71: 189–194. [DOI] [PubMed] [Google Scholar]
- 6. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ 2018; 362: k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao B, Zhao H, Zhao J. Impact of clinicopathological characteristics on survival in patients treated with immune checkpoint inhibitors for metastatic melanoma. Int J Cancer 2019; 144: 169–177. [DOI] [PubMed] [Google Scholar]
- 8. Zhang S, Liang F, Li W, et al. Risk of treatment-related mortality in cancer patients treated with ipilimumab: a systematic review and meta-analysis. Eur J Cancer 2017; 83: 71–79. [DOI] [PubMed] [Google Scholar]
- 9. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carbone DP, Reck M, Paz-Ares L, Creelan B, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461–2471. [DOI] [PubMed] [Google Scholar]
- 13. Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016; 375: 1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 16. Zhao B, Zhao H, Zhao J. Serious adverse events and fatal adverse events associated with nivolumab treatment in cancer patients: nivolumab-related serious/fatal adverse events. J Immunother Cancer 2018; 6: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdel-Rahman O, Helbling D, Schmidt J, et al. Treatment-related death in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Clin Oncol (R Coll Radiol) 2017; 29: 218–230. [DOI] [PubMed] [Google Scholar]
- 18. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008; 61: 64–75. [DOI] [PubMed] [Google Scholar]
- 22. Brok J, Thorlund K, Gluud C, et al. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008; 61: 763–769. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 26. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017; 377: 1824–1835. [DOI] [PubMed] [Google Scholar]
- 27. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377: 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015; 16: 375–384. [DOI] [PubMed] [Google Scholar]
- 29. Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in checkmate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2018; 36: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387: 1837–1846. [DOI] [PubMed] [Google Scholar]
- 31. Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018; 391: 748–757. [DOI] [PubMed] [Google Scholar]
- 32. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. New Engl J Med 2018; 378: 1789–1801. [DOI] [PubMed] [Google Scholar]
- 35. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 36. Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018; 392: 123–133. [DOI] [PubMed] [Google Scholar]
- 37. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 38. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16: 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017; 390: 1853–1862. [DOI] [PubMed] [Google Scholar]
- 40. Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity 2018; 48: 434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 2018; 363: k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang DY, Ye F, Zhao S, et al. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: a systematic review and meta-analysis. Oncoimmunology 2017; 6: e1344805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018; 360: k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med 2015; 175: 274–286. [DOI] [PubMed] [Google Scholar]
- 45. Zhao B, Zhao H. Impact of clinicopathological characteristics on the efficacy of neoadjuvant therapy in patients with human epidermal growth factor receptor-2-positive breast cancer. Int J Cancer 2018; 142: 844–853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, suppl_material for Fatal adverse events associated with programmed cell death protein 1 or programmed cell death-ligand 1 monotherapy in cancer by Bin Zhao, Hong Zhao and Jiaxin Zhao in Therapeutic Advances in Medical Oncology