Abstract
Background
Despite modern imaging modalities, staging of clinically staged T2N0M0 (cT2N0M0) oesophageal cancer is suboptimal, often leading to overtreatment. Endoscopic resection – the first-line therapy for early localised tumours – could be used to improve staging and to attain predictors of nodal upstaging enabling more stage-guided treatment decisions.
Objective
A systematic literature review and a meta-analysis were conducted to assess the prevalence and the pathological risk factors of lymph node metastases in cT2N0M0 oesophageal cancer.
Methods
Databases of PUBMED, EMBASE and Cochrane were searched for literature. The primary outcome was lymph node metastases determined after primary surgical resection.
Results
Nine studies with a total of 1650 cT2N0M0 patients were included. The prevalence of lymph node metastases was 43% (95% confidence interval: 35–50%) with heterogeneity being high across studies (I2 = 0.86, p < 0.001). Factors potentially attainable by endoscopic resection and having a significant association with lymph node metastases were invasion depth, differentiation grade, tumour size, depth of invasion in the muscularis propria and lymphovascular invasion.
Conclusions
Clinical lymph node staging is inaccurate in almost half of cT2N0M0 oesophageal cancer. Endoscopic resection is a promising diagnostic modality that might even be a valid alternative to surgery in selected patients without high-risk features, but further evidence is warranted.
Keywords: Endoscopic resection, lymph node metastasis, cancer staging, oesophagus, early cancer
Introduction
Oesophageal cancer yearly accounts for over half a million new cases worldwide.1 The incidence of oesophageal adenocarcinoma is rapidly rising in Western countries.1,2 Over the last two decades, neoadjuvant chemoradiotherapy has been successfully introduced in the curative treatment of oesophageal cancer leading to significant improvement of survival and quality of life.3–7 Five-year overall survival rates have been reported to barely exceed 20–40% after upfront oesophagectomy.5,8,9
Since the publication of the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) in 2012, there has been consensus that neoadjuvant therapy followed by oesophagectomy is more beneficial than surgical resection alone for locally advanced resectable oesophageal cancer.5 For early localised tumours (T1a-1b), endoscopic resection (ER) is considered first-line therapy.10
The prognosis of oesophageal cancer is much affected by lymph node metastases (LNM) which are associated with poorer survival.11 The advantage of neoadjuvant therapy is mainly in its potential to induce tumour regression and treat occult micrometastatic disease, improving the resectability rate and survival outcome.12–14 Patients with locally advanced tumours (T3-T4) treated with neoadjuvant therapy followed by surgical resection have improved survival compared to surgical resection alone.5,7 However, the survival benefit of neoadjuvant chemoradiotherapy for clinically staged T2N0M0 (cT2N0M0) disease is controversial. A recent meta-analysis showed that both treatment strategies were comparable in terms of post-operative complications and long-term survival.15
Recommendations on the optimal treatment for unselected cT2N0M0 tumours is complicated by suboptimal clinical staging and a small number of included patients with cT2 in randomised controlled trials.5,16–18 Despite early-stage disease, accurate staging is achieved in only 13–40% of cT2N0M0 tumours.19,20 Pathological Upstaging (30–50%) mostly due to presence of LNM, and pathological downstaging (20–55%) are common problems.20–24 Due to this inaccuracy, there is a tendency to recommend neoadjuvant therapy to patients with cT2N0M0 secondary to the risk of nodal upstaging. Theoretically, this strategy results in overtreatment and considerable morbidity as surgery alone, preferably without lymphadenectomy, or ER would be sufficient in approximately half of patients.
Due to the diagnostic limitations of current imaging modalities to detect LNM, therapeutic ER of unselected cT2N0M0 oesophageal cancer is generally not recommended. However, minimally invasive ER is an effective diagnostic tool providing determination of histopathological tumour characteristics. The supplementary staging information attained by ER combined with the primary findings from imaging might enhance accurate tumour staging of cT2N0M0 cancer. In addition, reliable risk prediction of LNM based on pathological factors determined by ER could enable more stage-guided treatment decisions. After careful histopathological assessment of ER specimens, endoscopic therapy might even be considered an effective therapeutic alternative to surgery in selected cT2N0M0 patients with predicted low risk of LNM. Pathological predictors of nodal upstaging that are attainable by ER are of particular interest to assess the risk of LNM and to determine whether ER suffices as the therapeutic modality in selected patients.
In the last decade, several studies have been performed focusing on histopathological risk factors for LNM. However, the number of these studies in T2 tumours is limited compared to T1 tumours.25–29 In this study, we performed a systematic review of the literature and provide an overview of pathological risk factors that are associated with the presence of LNM in cT2N0M0 oesophageal cancer.
Methods
Literature search
The electronic databases of PUBMED, EMBASE and Cochrane were systematically reviewed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines from 2000 up to December 2017 for possible articles.30 The review protocol was registered in PROSPERO (registration number: CRD42019127657). A qualified librarian assisted in conducting a comprehensive literature search strategy using the MESH terms ‘(o)esophagus’, ‘cancer’ and ‘lymph nodes’ (the full search strategy is presented in the Supplementary Material). Two independent authors (AAK and JH) assessed the resulting studies by title and abstract to determine if the inclusion and exclusion criteria were met. The reference lists of included articles were additionally screened for additional studies.
Inclusion and exclusion criteria
The search was restricted to published human studies written in English. Studies were included if data regarding LNM in chemoradiotherapy-naïve cT2N0M0 patients was reported separately related to pathological variables. Because we were also interested in pathological factors that might be useful in histopathological assessment of ER specimens, we also selected studies with pathologically staged cancer (pT2). Results from pT2 cases are presented separately from cT2 cases. Primary endpoint was pathologically confirmed LNM after surgical resection. Studies were excluded if nodal involvement was not analysed in an association or regression model, and if relevant raw data could not be extracted to calculate the association. Conference abstracts, reviews, letters to the editor and case reports were also excluded. In the case of overlap in patient populations, only the study with the most updated data was selected for inclusion.
Data extraction and quality assessment
Study characteristics that were collected included first author, year of publication, journal of publication, location where the study was performed and time period. To assess the generalisability of included studies, we also extracted study design, study population, inclusion criteria, method(s) of tumour staging and total number of patients included.
Critical appraisal of the studies was done using the Newcastle-Ottawa scale, assessing patient selection methods, comparability of study groups and outcome.31 Studies with six or more stars (on a scale of 0–8) were considered as good quality research. Disagreements between the two independent authors concerning the quality of studies or extracted data were resolved after discussion with the senior author (PDS).
Statistical analysis
Proportion meta-analysis was performed on pooled data using a random effects model (MedCalc version 18). Due to suboptimal quality of reporting and heterogeneity of included pathological factors, extraction of the number of patients with LNM related to specific factors was not possible for all included studies. Therefore, a complete data synthesis for predictors of LNM could not be performed. If a factor was reported in more than one study the effect measure was extracted from univariate analysis or raw numbers from a baseline table and was pooled when possible to summarise its predictive effect. For the proportion of patients with LNM a 95% confidence interval (CI) was estimated. In the case of continuous data, the difference between observed means was calculated with a 95% CI. For categorial data the effect size is presented as odds ratio (OR) with a 95% CI.
Results
Study selection
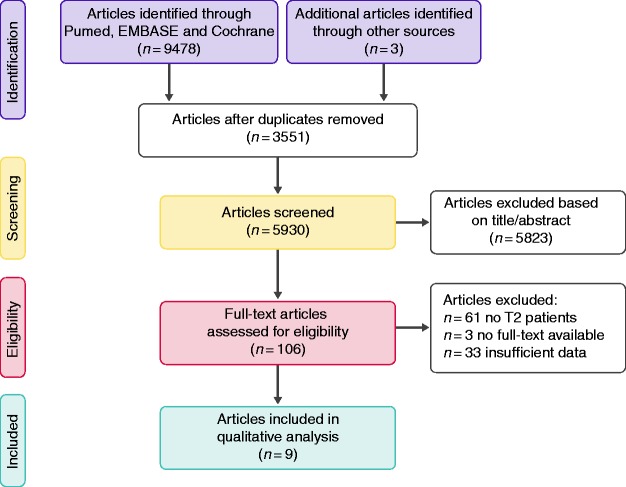
The initial search strategy resulted in 9478 articles, including three additional articles identified through other sources (Figure 1). After exclusion of duplicate publications, 5930 articles were screened, of which 5823 articles were excluded based on title and abstract. The remaining 106 possibly eligible articles were subjected to full-text assessment. Excluded studies included 61 studies without T2 patient population, 33 with insufficient histopathological data and three without full-text available. In total, nine studies were included in the present study.
Figure 1.
Flow diagram of included studies.
Study characteristics
All nine studies included in the review were retrospective in design. Of these studies, seven contained data from surgical cohorts of patients with T2 oesophageal cancer and three studies were based on data from national registries. The main characteristics of all studies are outlined in Table 1. A total of 1855 unique patients with a cT2 or pT2 tumour undergoing primary surgical resection were included (Table 2). Two studies combined both cT1N0 and cT2N0 tumours as one group in regression analyses of risk factors.23,32 Two explorative studies on the prognostic significance of pT2 tumour invasion depth into the muscularis propria were also selected for review. All studies reported data on upstaging after surgical resection as endpoint. If only upstaging is reported with no further indication on whether it was based on pT or pN category, we specified this in the analysis. Cases treated with neoadjuvant treatment were excluded from the analysis.
Table 1.
General characteristics of included studies.
| First author | Year | Location | Study design | Setting | Period | Study population | Histology | Oesophagectomy | Neoadjuvant therapy | Clinical staging method |
|---|---|---|---|---|---|---|---|---|---|---|
| Brown32 | 2017 | USA | Retrospective | National database | 2010–2013 | cT1-2N0M0 | AC | Yes | No | NR |
| Duan38 | 2017 | China | Retrospective | Cohort | 2006–2011 | pT2N0M0 | SCC | Yes | No | Biopsy proven |
| Samson33 | 2016 | USA | Retrospective | National database | 2006–2012 | cT2N0M0 | AC/SCC | Yes | Yes/No | NR |
| Shin23 | 2014 | South Korea | Retrospective | Cohort | 2005–2010 | cT1-2N0M0 | AC/SCC | Yes | No | EUS + PET-CT |
| Guo39 | 2014 | China | Retrospective | Cohort | 2008–2013 | pT2N0 | SCC | Yes | No | EUS + CT |
| Hardacker37 | 2014 | USA | Retrospective | Cohort | 1990–2011 | cT2N0M0 | AC/SCC | Yes | Yes/No | EUS + cross-sectional imaging |
| Crabtree34 | 2013 | USA | Retrospective | National database | 2002–2011 | cT2N0 | AC/SCC | Yes | Yes/No | Biopsy proven |
| Gaur36 | 2010 | USA | Retrospective | Cohort | 1995–2007 | cT1-4 N0-3 M0 | AC | Yes | No | Endoscopic biopsy + EUS with FNA + CT/EUS/PET-CT |
| Kunisaki35 | 2010 | Japan | Retrospective | Cohort | 1992–2005 | cT1-4 N0-1 M0 | SCC | Yes | No | Barium swallow X-ray + endoscopic biopsy + CT |
AC: adenocarcinoma; CT: computed tomography; EUS: endoscopic ultrasound; NR: not reported; PET: positron emission tomography; SCC: squamous cell carcinoma.
Table 2.
The proportion of patients with lymph node metastases (LNM).
| Study | Study sample size | Number of interest (T2N0M0) | LNM+, n (%) | 95% CI for proportion | Male, n (%) | Age |
|---|---|---|---|---|---|---|
| Brown 201732 | 1120 | 270 | 87 (32%) | 0.27–0.38 | 972 (87%) | Mean 64 ± 9 |
| Duan 201738 | 120 | 120a | 37 (45%) | 0.34–0.56 | NR | NR |
| Samson 201633 | 1785 | 713 | 239 (34%) | 0.30–0.70 | 587 (82%) | Mean 65.6 ± 10.9 |
| Shin 201423 | 240 | 66 | 26 (39%) | 0.28–0.52 | 228 (95%) | Mean 63.1 (39–80) |
| Guo 201439 | 85 | 85a | 36 (42%) | 0.32–0.54 | 63 (74 %) | Mean 59.9 ± 7.9 |
| Hardacker 201437 | 68 | 35 | 14 (40%) | 0.24–0.58 | 55 (81%) | Median 61.7, IQR NR |
| Crabtree 201334 | 752 | 482 | 184 (38%) | 0.34–0.43 | 626 (82%) | Mean 63.8 ± 11.1 |
| Gaur 201036 | 164 | 34 | 18 (53%) | 0.35–0.70 | 140 (85%) | Mean 60 ± 16.7 |
| Kunisaki 201035 | 210 | 50 | 38 (76%) | 0.62–0.87 | 174 (83%) | Mean 62.9 ± 8.1 |
| Total (T2N0M0) | 1855 | |||||
| Total (cT2N0M0) | 1650 |
CI: confidence interval; IQR: interquartile range; LNM: lymph node metastases; NR: not reported.
pT2 tumours, not included in meta-analysis of proportions.
Prevalence of LNM
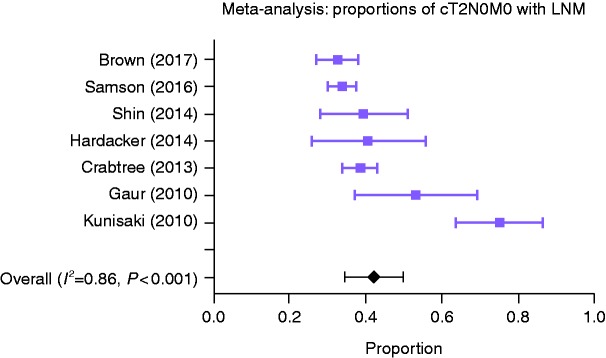
The identified studies included a total of 1855 patients with T2N0M0 oesophageal cancer (Table 2), of which there were 1650 patients with cT2N0M0 in seven studies. The reported proportion of LNM varied largely between studies, from 32–76%. Only the cT2N0M0 group was included in the meta-analysis of proportions. Due to the high heterogeneity (I2 = 0.86, p < 0.001), the random effects model of meta-analysis was preferred. In the pooled proportion of patients, the prevalence of LNM was 43% (95% CI: 35–50%). Figure 2 shows a forest plot of reported and pooled proportions of patients with LNM. By judging proportions from the oldest to the most recent studies, it seems that the clinical staging has improved over time with decreasing proportions of LNM in cT2N0M0.
Figure 2.
Forest plot of proportions of cT2N0M0 patients with lymph node metastases (LNM) of the different studies with pooled proportions (random effects model, 95% confidence interval (CI)).
Clinical staging method
The included studies showed inconsistency in reported clinical staging methods (Table 1). Data on staging modalities were not available in the three registry-based studies.32–34 Only three studies reported details of clinical staging methods.23,35,36
Quality assessment
All included studies scored fair to good on the Newcastle-Ottawa Scale (Supplementary Material Table 1). In four studies comparability could not be assessed because of lacking data. Since the endpoint was pathological LNM at surgery, the follow-up in all studies was assessed to be long enough for the outcome. Clinicopathological variables were retrieved from institutional patient records or database registries. Four studies had pathological upstaging as endpoint, which included pT, as well as pN category upstaging.32–34,37
Predictive factors for LNM
Table 3 shows all identified pathological factors in the different studies with the reported or calculated risk of LNM based on univariate analyses. Factors with a significant association in more than one study were also included in a separate table (Supplementary Material Tables 2–5). These factors were tumour differentiation grade (poor vs well), number of resected lymph nodes, depth of invasion into the muscularis propria and pT category (pT2 vs pT1).
Table 3.
Overview of pathological factors in relation to lymph node metastases based on reported or calculated univariate analyses.
| Risk factors | Brown 2017 | Duan 2017 | Samson 2016 | Shin 2014 | Hardacker 2014 | Guo 2014 | Crabtree 2013 | Gaur 2010 | Kunisaki 2010 |
|---|---|---|---|---|---|---|---|---|---|
| Differentiation: well vs poor | – | – | X | X | X | – | – | – | – |
| Number resected lymph nodes | Xa | – | X | – | – | – | – | – | – |
| Clinical T category: cT1 vs cT2 | X | – | – | X | – | – | – | X | – |
| Pathological T category: pT1 vs pT2 | X | – | – | – | – | – | – | – | X |
| Tumour size | Xb | – | Oc | Od | Oc | – | – | – | – |
| Histology type: AC vs SCC | – | – | O | O | – | – | – | – | – |
| Depth invasion in muscularis propria | – | X | – | – | – | X | – | – | – |
| Tumour location: upper/middle/lower third | O | – | – | O | – | – | – | – | – |
| Lymphovascular invasion | – | – | X | – | – | – | – | – | – |
| Positive surgical margins | X | – | – | – | – | – | – | – | – |
AC: adenocarcinoma; SCC: squamous cell carcinoma.
Factors significantly associated with the risk of LNM are marked with an X. Included factors that were non-significant are marked with an O.
aNo.:<10, 10–15, 16–25 and >25; bsize: >1 cm, 1.1–3.0, 3.1–5.0 and >5 cm; cmean tumour size; dtumour size <2 cm vs >2 cm.
Tumour differentiation grade
Three studies analysed tumour differentiation in relation to the risk of LNM.23,33,37 For this review, differentiation was classified as well or poor. All three studies concluded that a poor differentiation was associated with a higher risk of LNM with ORs of 4.8 (95% CI: 1.1–21.4), 5.7 (95% CI: 1.5–21.1), and 8.7 (95% CI: 3.8–19.9), respectively (Supplementary Material Table 2). Pooling the data was not possible because in one study the endpoint was pathological upstaging of pT and pN category combined.37 Another study analysed cT1–cT2 cases as one group, providing no possibility to separate of both categories based on raw numbers.23
Number of resected lymph nodes
Adequate lymphadenectomy during surgery is crucial to assess the presence of LNM. In two studies the number of resected lymph nodes was included (Supplementary Material Table 3). Samson et al. reported a significant mean difference of 3.7 (95% CI: 2.1–5.4) in the number of nodes between upstaged and downstaged patients, respectively.33 Brown et al. categorised the number of lymph nodes in four groups (no. <10, 10–15, 16–25 and >25 nodes) and found that a higher number of examined nodes (>10) was associated with an increased OR for upstaging (Supplementary Material Table 3).32
Pathological tumour category
Pathological upstaging to pT3–pT4 was reported in 17–40% of cT2N0M0 cases. When pathological tumour category, representing depth of tumour invasion into the mucosal wall, was classified as pT2 versus pT1, Brown et al. and Kunisaki et al. concluded that pT2 tumours had a higher likelihood of LNM with OR 6.4 (95% CI: 4.2–9.986) and OR 8.4 (95% CI: 3.6–19.3), respectively, compared to pT1 (Supplementary Material Table 4).32,35
Depth of invasion in the muscularis propria
Two studies investigated tumour invasion depth into the muscularis propria as risk factor for LNM in pT2 squamous cell carcinoma.38,39 Depth of invasion into the muscularis propria was classified as invasion into the outer longitudinal versus the inner circular muscle layer. Both Duan et al. and Guo et al. reported that pT2 tumours invading into the longitudinal layer were at an increased risk for LNM with OR 2.5 (95% CI: 1.0–6.2) and OR 2.5 (95% CI: 1.0–6.0), respectively (Supplementary Material Table 5).
Additional risk factors
Some additional risk factors were reported (Table 3). These factors included tumour size, type of histology, tumour location, tumour length, lymphovascular invasion (LVI) and positive surgical margins. However, because each of these factors was reported in only one study or a significant association was not found in multiple studies, no effect sizes are presented here.
Discussion
The pooled proportion of cT2N0M0 patients with LNM in this meta-analysis was 43%. This finding is generally in line with international multicentre and large population-based studies reporting similar rates for cT2N0M0 cohorts.19,32,34,40,41 However, in relatively small cohort studies nodal upstaging rates up to 65% have been reported.21,35 The differences in LNM prevalence between studies are most likely due to small sample size and insufficient sensitivity of clinical staging methods used. Although we did not perform a trend analysis, clinical staging accuracy appeared to improve over time. In a recent multicentre study including 499 cT2N0M0 patients treated with primary oesophagectomy, clinical staging accuracy did not improve over a period of 10 years (2002–2012).41 Our observation might actually be the effect of increased experience with modern imaging modalities, as included patients were from an extended study period (1990–2013).
The significant proportion of cT2N0M0 patients with LNM after primary surgical resection (43%) highlights the limitations of current imaging modalities. Despite increased experience with endoscopic ultrasound (EUS) and positron emission tomography-CT, in the era of neoadjuvant therapy, the staging accuracy at this tumour stage is not yet satisfactory.42 One of the great challenges of current imaging modalities is to detect lymph node micrometastasis.43 In cT2N0M0 patients treated with neoadjuvant chemoradiotherapy, the observed rate of LNM in the surgical resection specimen also remains remarkably high, ranging from 20–50%.18,41,44 As an explanation, it has been suggested that the discrepancy between the cN and pN category might be explained by tumour progression during the waiting time to surgery which was previously not detected at baseline imaging.45 However, this seems unlikely as longer waiting times to surgery (>2 weeks) have not been shown to affect the correlation between cN and pN.45
Despite considerable inconsistency in methodology and reported outcomes of the studies, we identified a number of factors associated with increased risk of LNM. Pathological factors that showed a significant association with LNM in at least one study were: poor differentiation, pT category, larger tumour size, invasion of the longitudinal muscle layer of the muscularis propria, LVI, higher number of examined lymph nodes and positive surgical resection margins. Tumour differentiation grading, depth of invasion, tumour size, depth of invasion in the muscularis propria and LVI are all factors that are easily attainable by ER. Unfortunately, the level and power of evidence is still too low to allow firm treatment recommendations for cT2N0M0 cancer based on reliable clinical risk prediction of nodal upstaging. In the context of a future clinical risk prediction tool, especially variables that can be evaluated in the ER specimens and maybe also in biopsies, such as differentiation grade, pT category, tumour size and LVI, are interesting. In a recent decision analysis model, it was concluded that cT2N0M0 patients with higher tumour grade, tumour size >3 cm or LVI were at an increased risk of upstaging and would gain a survival benefit from neoadjuvant chemoradiotherapy.46 Another multicentre study came to similar conclusions.41
The management of cT2N0M0 oesophageal cancer presents clinicians with a dilemma. Current guidelines are ambiguous with regard to advising neoadjuvant therapy for unselected cT2N0M0 tumours.3,4 In the CROSS trial of 2012, cT2 tumours made up only 15% of all tumours (26/178) in the neoadjuvant chemoradiotherapy followed by surgery group, making extrapolation of the survival benefit of neoadjuvant treatment to this particular substage questionable.5 Multiple studies focusing on the benefit of neoadjuvant chemoradiotherapy for patients with cT2N0M0 oesophageal cancer have remained equivocal. Two population-based studies, from the USA and the Netherlands respectively, demonstrated significantly improved overall survival after neoadjuvant therapy in cT2N0M0 staged patients.19,33 However, two recent meta-analyses did not show a survival benefit compared to surgery alone.15,47 Using the Surveillance Epidemiology and End Results (SEER) database, Song et al. reported that in cT2-T3N0M0 staged patients only T3N0M0 tumours had a survival benefit from neoadjuvant chemoradiotherapy.48 In a phase III randomised clinical trial by Mariette et al., neoadjuvant chemoradiotherapy did not improve overall survival of stage I/II, oesophageal cancer (n = 110/195 (56%) cT2), but did increase in-hospital postoperative mortality.17 Besides a potential increase in morbidity and mortality, neoadjuvant treatment is also associated with increased treatment costs.17,49
In view of the potential consequences of neoadjuvant therapy and radical surgery as first line treatment in unselected cT2N0M0 cancer, progress in the area of endoscopic interventions for early-staged oesophageal cancer might be of great importance to improve the staging and risk assessment of cT2N0M0 cancer. ER is, in fact, an established curative treatment for cT1 oesophageal adenocarcinoma with low-risk histopathological characteristics in the ER specimen.10 Further work is required to establish the viability of expanding ER indications for management of cT2N0M0, similar to cT1 cancer. To date, only two small studies have investigated the diagnostic and therapeutic utility of endoscopic mucosal resection (EMR) for cT2N0M0 cancer. In both studies, reassessment of selected EUS-staged cT2N0M0 patients treated by EMR resulted in downstaging of 40–50% of the tumours in which curative ER was also achieved with no major complications.50,51 In future investigations, emphasis on high-risk histopathological and/or molecular markers for LNM that could be assessed in the ER specimen is needed to enable stage-guided treatment decisions, i.e. organ-sparing endoscopic treatment versus radical surgical treatment.52,53
In this systematic review, we report several candidate predictors for LNM in cT2N0M0 oesophageal cancer that warrant further investigation. However, the present study comes with some considerable limitations. First, the absence of data from randomised trials is a major limitation as retrospective studies are prone to confounding. Second, two studies reported logistic regression for associations with LNM in cT1-2N0M0 cancer. The known number of cT1 patients could be omitted in the meta-analysis but this was not possible when evaluating the risk factors associated with LNM. The addition of early-stage cT1 cases to the more advanced cT2 cases inevitably downstages the group which might have weakened the association analysis. Third, because each study reported different histopathological parameters and endpoints (pN vs pT-pN upstaging), we were not able to perform a comprehensive data synthesis based on pooled data. Finally, only data from univariate analysis was used in order to increase the comparability of the studies. However, the use of multivariate analysis with correction for probable confounding factors is obviously needed to identify independent risk factors for LNM.
In conclusion, clinical lymph node staging remains highly inaccurate in cT2N0M0 oesophageal cancer with almost half of patients being diagnosed with LNM in final pathology. Due to staging inaccuracy, there is a tendency to recommend radical surgery with or without neoadjuvant therapy to these patients. However, this approach is controversial since, on the other hand, more than 50% of patients do not have LNM and/or are downstaged. We hypothesise that minimally invasive endoscopic treatment strategies, such as EMR, offer diagnostic opportunities for improved staging and risk assessment, directing more stage-guided treatment selection. In selected cT2N0M0, with no high-risk features in the ER specimen, ER could likely be considered therapeutic, avoiding overtreatment by radical surgery or neoadjuvant therapy in these patients. However, reliable histopathological predictors for the risk of LNM are vital for an effective endoscopic strategy. Future studies on current topics are therefore recommended.
Supplemental Material
Supplemental material, UEG879007 Supplemental Material for Predicting lymph node metastases with endoscopic resection in cT2N0M0 oesophageal cancer: A systematic review and meta-analysis by Ali Al-Kaabi, Rachel S van der Post, Jonathan Huising, Camiel Rosman, Iris D Nagtegaal and Peter D Siersema in United European Gastroenterology Journal
Declaration of conflicting interests
There were no conflicts of interest for any of the authors.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Wong MCS, Hamilton W, Whiteman DC, et al. Global incidence and mortality of oesophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries. Sci Rep 2018; 8: 4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold M, Laversanne M, Brown LM, et al. Predicting the future burden of esophageal cancer by histological subtype: International trends in incidence up to 2030. Am J Gastroenterol 2017; 112: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 3.Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27: v50–v57. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw 2011; 9: 830–887. [DOI] [PubMed] [Google Scholar]
- 5.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 6.Noordman BJ, Verdam MGE, Lagarde SM, et al. Impact of neoadjuvant chemoradiotherapy on health-related quality of life in long-term survivors of esophageal or junctional cancer: Results from the randomized CROSS trial. Ann Oncol 2018; 29: 445–451. [DOI] [PubMed] [Google Scholar]
- 7.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008; 26: 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: A randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg 1992; 16: 1104–1109. discussion: 1110. [DOI] [PubMed] [Google Scholar]
- 9.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol 2005; 6: 659–668. [DOI] [PubMed] [Google Scholar]
- 10.Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 2017; 49: 191–198. [DOI] [PubMed] [Google Scholar]
- 11.Cho JW, Choi SC, Jang JY, et al. Lymph node metastases in esophageal carcinoma: An endoscopist's view. Clin Endosc 2014; 47: 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulshof MC, van Laarhoven HW. Chemoradiotherapy in tumours of the oesophagus and gastro-oesophageal junction. Best Pract Res Clin Gastroenterol 2016; 30: 551–563. [DOI] [PubMed] [Google Scholar]
- 13.Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol 2013; 3: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davarzani N, Hutchins GGA, West NP, et al. Prognostic value of pathological lymph node status and primary tumour regression grading following neoadjuvant chemotherapy – results from the MRC OE02 oesophageal cancer trial. Histopathology 2018; 72: 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mota FC, Cecconello I, Takeda FR, et al. Neoadjuvant therapy or upfront surgery? A systematic review and meta-analysis of T2N0 esophageal cancer treatment options. Int J Surg 2018; 54: 176–181. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 17.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014; 32: 2416–2422. [DOI] [PubMed] [Google Scholar]
- 18.Markar SR, Gronnier C, Pasquer A, et al. Role of neoadjuvant treatment in clinical T2N0M0 oesophageal cancer: Results from a retrospective multi-center European study. Eur J Cancer 2016; 56: 59–68. [DOI] [PubMed] [Google Scholar]
- 19.Goense L, Visser E, Haj Mohammad N, et al. Role of neoadjuvant chemoradiotherapy in clinical T2N0M0 esophageal cancer: A population-based cohort study. Eur J Surg Oncol 2018; 44: 620–625. [DOI] [PubMed] [Google Scholar]
- 20.Rice TW, Mason DP, Murthy SC, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg 2007; 133: 317–324. [DOI] [PubMed] [Google Scholar]
- 21.Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: The risk of node positive disease. Ann Thorac Surg 2011; 92: 491–496. discussion 496–498. [DOI] [PubMed] [Google Scholar]
- 22.Markar SR, Gronnier C, Pasquer A, et al. Discrepancy between clinical and pathologic nodal status of esophageal cancer and impact on prognosis and therapeutic strategy. Ann Surg Oncol 2017; 24: 3911–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin S, Kim HK, Choi YS, et al. Clinical stage T1-T2N0M0 oesophageal cancer: Accuracy of clinical staging and predictive factors for lymph node metastasis. Eur J Cardiothorac Surg. 2014; 46: 274–279; discussion 279. [DOI] [PubMed] [Google Scholar]
- 24.Speicher PJ, Ganapathi AM, Englum BR, et al. Induction therapy does not improve survival for clinical stage T2N0 esophageal cancer. J Thorac Oncol 2014; 9: 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim DU, Lee JH, Min BH, et al. Risk factors of lymph node metastasis in T1 esophageal squamous cell carcinoma. J Gastroenterol Hepatol 2008; 23: 619–625. [DOI] [PubMed] [Google Scholar]
- 26.Weksler B, Kennedy KF, Sullivan JL. Using the National Cancer Database to create a scoring system that identifies patients with early-stage esophageal cancer at risk for nodal metastases. J Thorac Cardiovasc Surg 2017; 154: 1787–1793. [DOI] [PubMed] [Google Scholar]
- 27.Dubecz A, Kern M, Solymosi N, et al. Predictors of lymph node metastasis in surgically resected T1 esophageal cancer. Ann Thorac Surg 2015; 99: 1879–1885. discussion: 1886. [DOI] [PubMed] [Google Scholar]
- 28.Moon JY, Kim GH, Kim JH, et al. Clinicopathologic factors predicting lymph node metastasis in superficial esophageal squamous cell carcinoma. Scand J Gastroenterol 2014; 49: 589–594. [DOI] [PubMed] [Google Scholar]
- 29.Lee L, Ronellenfitsch U, Hofstetter WL, et al. Predicting lymph node metastases in early esophageal adenocarcinoma using a simple scoring system. J Am Coll Surg 2013; 217: 191–199. [DOI] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells G, Shea B, O'Connell D, et al. The NewcastleOttawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (last accessed: 4 April 2018).
- 32.Brown CS, Gwilliam N, Kyrillos A, et al. Predictors of pathologic upstaging in early esophageal adenocarcinoma: Results from the national cancer database. Am J Surg 2018; 216: 124–130. [DOI] [PubMed] [Google Scholar]
- 33.Samson P, Puri V, Robinson C, et al. Clinical T2N0 esophageal cancer: Identifying pretreatment characteristics associated with pathologic upstaging and the potential role for induction therapy. Ann Thorac Surg 2016; 101: 2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crabtree TD, Kosinski AS, Puri V, et al. Evaluation of the reliability of clinical staging of T2 N0 esophageal cancer: A review of the Society of Thoracic Surgeons database. Ann Thorac Surg 2013; 96: 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunisaki C, Makino H, Oshima T, et al. Clinicopathological features in N0 oesophageal cancer patients. Anticancer Res 2010; 30: 3063–3069. [PubMed] [Google Scholar]
- 36.Gaur P, Sepesi B, Hofstetter WL, et al. A clinical nomogram predicting pathologic lymph node involvement in esophageal cancer patients. Ann Surg 2010; 252: 611–617. [DOI] [PubMed] [Google Scholar]
- 37.Hardacker TJ, Ceppa D, Okereke I, et al. Treatment of clinical T2N0M0 esophageal cancer. Ann Surg Oncol 2014; 21: 3739–3743. [DOI] [PubMed] [Google Scholar]
- 38.Duan J, Deng T, Ying G, et al. Prognostic significance of the T2 substage in patients with esophageal squamous cell carcinoma. Dis Esophagus 2017; 30: 1–7. [DOI] [PubMed] [Google Scholar]
- 39.Guo W, Xiao HL, Ma Z, et al. Should stage T2 esophageal squamous cell carcinoma be subclassified?. Ann Surg Oncol 2014; 21: 2540–2545. [DOI] [PubMed] [Google Scholar]
- 40.Shridhar R, Huston J, Meredith KL. Accuracy of endoscopic ultrasound staging for T2N0 esophageal cancer: A National Cancer Database analysis. J Gastrointest Oncol 2018; 9: 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esophageal Cancer Study Group Participating Centers. Predictors of staging accuracy, pathologic nodal involvement, and overall survival for cT2N0 carcinoma of the esophagus. J Thorac Car-diovasc Surg 2019; 157: 1264–72. [DOI] [PubMed] [Google Scholar]
- 42.van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: A meta-analysis. Br J Cancer 2008; 98: 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fencl P, Belohlavek O, Harustiak T, et al. FDG-PET/CT lymph node staging after neoadjuvant chemotherapy in patients with adenocarcinoma of the esophageal-gastric junction. Abdom Radiol (NY) 2016; 41: 2089–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goense L, van Rossum PS, Kandioler D, et al. Stage-directed individualized therapy in esophageal cancer. Ann N Y Acad Sci 2016; 1381: 50–65. [DOI] [PubMed]
- 45.Fisher JM, Pohl H, Gordon SR, et al. The impact of time elapsed between endoscopic ultrasound and esophagectomy on concordance of ultrasonographic and pathologic staging of esophageal malignancy. Dig Dis Sci 2011; 56: 2987–2991. [DOI] [PubMed] [Google Scholar]
- 46.Semenkovich TR, Panni RZ, Hudson JL, et al. Comparative effectiveness of upfront esophagectomy versus induction chemoradiation in clinical stage T2N0 esophageal cancer: A decision analysis. J Thorac Cardiovasc Surg. Epub ahead of print 13 February 2018. DOI: 10.1016/j.jtcvs.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lv HW, Xing WQ, Shen SN, et al. Induction therapy for clinical stage T2N0M0 esophageal cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2018; 97: e12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song Y, Tao G, Guo Q, et al. Survival benefit of surgery with radiotherapy vs surgery alone to patients with T2-3N0M0 stage esophageal adenocarcinoma. Oncotarget 2016; 7: 21347–21352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuppusamy M, Sylvester J, Low DE. In an era of health reform: Defining cost differences in current esophageal cancer management strategies and assessing the cost of complications. J Thorac Cardiovasc Surg 2011; 141: 16–21. [DOI] [PubMed] [Google Scholar]
- 50.Gotink AW, Spaander MCW, Doukas M, et al. Exploring diagnostic and therapeutic implications of endoscopic mucosal resection in EUS-staged T2 esophageal adenocarcinoma. Endoscopy 2017; 49: 941–948. [DOI] [PubMed] [Google Scholar]
- 51.Nelson DB, Mitchell KG, Weston BR, et al. Should endoscopic mucosal resection be attempted for cT2N0 esophageal cancer?. Dis Esophagus. Epub ahead of print 20 March 2019. DOI: 10.1093/dote/doz016. [DOI] [PubMed] [Google Scholar]
- 52.Kato K, Hida Y, Miyamoto M, et al. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer 2002; 94: 929–933. [PubMed] [Google Scholar]
- 53.Kurokawa T, Miyamoto M, Kato K, et al. Overexpression of hypoxia-inducible-factor 1alpha (HIF-1alpha) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br J Cancer 2003; 89: 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, UEG879007 Supplemental Material for Predicting lymph node metastases with endoscopic resection in cT2N0M0 oesophageal cancer: A systematic review and meta-analysis by Ali Al-Kaabi, Rachel S van der Post, Jonathan Huising, Camiel Rosman, Iris D Nagtegaal and Peter D Siersema in United European Gastroenterology Journal