Abstract
Polybrominated diphenyl ethers (PBDEs) are a group of brominated flame retardants that are ubiquitously detected in the environment and associated with adverse health outcomes. 6-OH-BDE-47 is a metabolite of the flame retardant, 2,2′,4,4′-Tetrabromodiphenyl ether (BDE-47), and there is increasing concern regarding its developmental neurotoxicity and endocrine disrupting properties. In this study, we report that early life exposure in zebrafish (Danio rerio) embryos to 6-OH-BDE-47 (50 and 100 nM) resulted in higher coiling frequency and significantly increased apoptotic cells in the brain. These effects were partially rescued by overexpression of thyroid hormone receptor β (THRβ) mRNA. Moreover, exposure to 100 nM 6-OH-BDE-47 significantly reduced the number of hypothalamic 5-hydroxytryptamine (5-HT, serotonin)-immunoreactive (5-HT-ir) neurons and the mRNA expression of tryptophan hydroxylase 2 (TPH2). These results indicate that 6-OH-BDE-47 affected thyroid hormone regulation through THRβ and negatively impacted the nervous system, in turn, affecting coiling behavior. Correlations of these endpoints suggest that coiling frequency could be used as an indicator of neurotoxicity in embryos.
Keywords: Polybrominated diphenyl ether (PBDE); Coiling; Apoptosis; Thyroid receptor (THRβ); 5-Hydroxytryptamnine (serotonin, 5-HT); Zebrafish
1. Introduction
For decades, polybrominated diphenyl ethers (PBDEs) have been added to commercial products such as flame retardants (Covaci et al., 2003). Even though most have been phased out of the market, PBDEs and their metabolites continue to be ubiquitously detected in environmental and human biological samples (Hites, 2004; Sjodin et al., 2008). This is due to their persistence and bioaccumulation properties as well as the fact that products impregnated with PBDEs remain in use (Darnerud et al., 2001). 2,2′,4,4′-Tetrabromodiphenyl ether (BDE-47) is one of the most abundant congeners of PBDEs found in the environment and potential adverse effects, including neurotoxicity, have gained attention. For example, epidemiological studies linked BDE-47 in cord blood to early childhood attention disorders (Cowell et al., 2015) and interference with cognitive function and behavior (Herbstman and Mall, 2014). Children with higher concentrations of BDE-47 had lower scores on tests of mental and physical development at 12 months (Herbstman et al., 2010). Recent studies showed that BDE-47 affected: spontaneous behavior (locomotion, rearing, and total activity), learning and memory functions in mice (Eriksson et al., 2001; Gee and Moser, 2008), and impaired long-term potentiation (LTP) in mouse hippocampus (Dingemans et al., 2007). This compound also induced oxidative stress and apoptosis in rat hippocampal neurons and mouse neuro-2a cells (He et al., 2008; Chen et al., 2017). Furthermore, BDE-47 caused inhibition of differentiation of human neural progenitor cells (hNPC) into neurons and oligodendrocytes, which was associated with decreased migration distance (Schreiber et al., 2010). However, the neurotoxicity of BDE-47 or its metabolites in aquatic organisms is largely unknown, and the underlying mechanisms for its neurotoxicity remain elusive.
6-hydroxy-2,2′,4,4′-Tetrabromodiphenyl ether (6-OH-BDE-47) is the hydroxyl-metabolic product of BDE-47, and its degradation half-life in the environment is 24 h (Liu et al., 2015). It has been detected in maternal serum and in umbilical cord blood of pregnant women in the US with median concentrations of 2.50 and 5.62 pg/mL, respectively (Stapleton et al., 2011; Chen et al., 2013a). Furthermore, the metabolite showed a stronger binding affinity to transthyretin (TTR) and thyroxine-binding globulin (TBG) in humans than its parent compound. 6-OH-BDE-47 also disturbed Ca2+ homeostasis and neurotransmitter release in rat PC12 cells at lower concentrations (Dingemans et al., 2008).
Few studies have reported on the effects of 6-OH-BDE-47 in aquatic organisms. In fish tissues sampled from the Detroit River between US and Canada, the concentrations of 6-OH-BDE-47 ranged between 1.5 and 10 pg/g (wet weight) (Valters et al., 2005). In surface sediment samples from freshwater Taihu Lake in China, the concentrations of 6-OH-BDE-47 ranged between <0.1 and 0.350 ng/g (dry weight) (Liu et al., 2017). In the laboratory, it induced developmental arrest in zebrafish embryos (Danio rerio) in a concentration-dependent manner (Usenko et al., 2011). Hydroxylated PBDEs have been shown to disrupt of endocrine systems of fish (Dong et al., 2014; Macaulay et al., 2015b). Mechanisms have been hypothesized to explain the endocrine disrupting effect of OH-PBDEs. First, OH-PBDEs compete with thyroxine (T4) for binding to TTR and thyroid hormone receptor β (THRβ), resulting in the disruption of thyroid homeostasis (Hamers et al., 2008; Ren and Guo, 2013). The second mechanism relates to the tendency of OH-PBDEs to inhibit the activity of thyroid sulfotransferase and deiodinase in vitro (Butt et al., 2011; Butt and Stapleton, 2013) and in vivo, leading to the disruption of thyroid hormone (TH) and energy metabolism during the zebrafish larval period (van Boxtel et al., 2008; Usenko et al., 2012; Legradi et al., 2014; Liu et al., 2015). Additionally, 6-OH-BDE-47 decreases THRβ mRNA expression, suggesting that it may be acting as a triiodothyronine (T3) mimic (Dong et al., 2014).
Exposure to 6-OH-BDE-47 or BDE-47 during early life stages of zebrafish might have profound effects in later life, including altered activity levels, habituation, fear/anxiety, and locomotor activity (Macaulay et al., 2015a). Behavior and movement are mediated by monoaminergic neurotransmitters including serotonin (5-hydroxytryptamine, 5-HT), dopamine (DP), noradrenaline (NE), and others. 5-HT orchestrates a broad array of processes including autonomic, cognitive, and behavioral functions, which regulate mood, appetite, memory, learning, pain, and establishment of left-right asymmetry in fish embryonic development (Gaspar et al., 2003; Asan et al., 2013). Impaired function of hypothalamic 5-hydroxytryptamine (5-HT, serotonin)-immunoreactive (5-HT-ir) neurons is related to a number of neurological disorders including depression, aggression, schizophrenia, and anxiety (Hendricks et al., 2003), synonymous to the phenotypes caused by 6-OH-BDE-47 in zebrafish (Macaulay et al., 2015a). In zebrafish, quipazine, a non-selective 5-HT agonist, increased the number of swimming episodes in larvae (Brustein et al., 2003), and fluoxetine, a selective 5-HT reuptake inhibitor, altered adult offensive aggressive behavior in dominant males attacking or chasing subordinates (Theodoridi et al., 2017). Dong et al. (2014) showed 6-OH-BDE-47 exposure of zebrafish embryos to increase apoptosis in the retina and reduce eye pigmentation. Should 6-OH-BDE-47 also induce apoptosis of 5-HT-ir neurons, there may be increased secretion of 5-HT via negative feedback. But effects of 6-OH-BDE-47 on 5-HT production and its rate-limiting enzyme, tryptophan hydroxylase 2 (TPH2), require more study.
6-OH-BDE-47 has potential for developmental neurotoxicity. It is closely related to human health, specifically children, and there are few mechanistic studies, particularly in very early stages of neurodevelopment. While there are several validated neurotoxicity tests for juveniles and adults (Padilla et al., 2011; Bailey et al., 2013; Macaulay et al., 2015a), few standard methods are available estimate early-life neurotoxicity. In this study, we exposed zebrafish embryos to various concentrations of 6-OH-BDE-47 and assessed coiling frequency as an indicator of neurotoxicity. We evaluated apoptosis in the brain and its links to thyroid function via the rescue effect of THRβ mRNA overexpression. The role of 5-HT in behavior and movement also made it a candidate for explaining changes in coiling frequency. Therefore, hypothalamic 5-hydroxytryptamine (5-HT, serotonin)-immunoreactive (5-HT-ir) neurons were evaluated. Our results provided insight into the mechanistic understanding of effects of 6-OH-BDE-47 on zebrafish embryo developmental neurotoxicity.
2. Materials and methods
2.1. Fish husbandry
Adult wild-type 5-D zebrafish (AB line) were maintained in a recirculating AHAB system (Aquatic Habitats, Apopka, FL, USA) according to standard protocols at 28 °C and a 14:10 light/dark photoperiod. Fish were fed twice daily with hatched brine shrimp and Zeigler’s Adult Zebrafish Complete Diet (Aquatic Habitats, USA). Embryos were collected after natural spawning in breeder tanks and then maintained in embryo media (5 mM NaCl, 0.33 mM MgSO4,0.33 mM CaCl2, and 0.17 mM KCl) (Macaulay et al., 2015a) in incubators under the same conditions as adults. All fish care and experimental procedures were approved by Duke University’s Institutional Animal Care & Use Committee.
2.2. Chemicals and exposure media
6-OH-BDE-47 (>99 .5% purity) was purchased from Accustandard (New Haven, CT, USA; HBDE-4005S-CN-0.2x). Stock solutions of 1 mM were prepared by dissolving an appropriate amount of 6-OH-BDE-47 in dimethylsulfoxide (DMSO; Sigma Aldrich, St. Louis, MO, USA; 472301–100 ML). Exposure solutions were prepared via serial dilution from stock solution using embryo rearing medium, resulting in a final concentration of DMSO less than 0.1%. The 4 different concentrations (1, 10, 50, and 100 nM) chosen for this study were based on those used in a previous study (Dong et al., 2014). The control group received 0.1% DMSO only. Exposure was from 4 h post fertilization (hpf) until 22, 26, 30, 34, or 96 hpf according to different experiments detailed below. Each treatment group contained 3 replicates (n = 3) of 10 embryos each.
2.3. Coiling behavior assay
Coiling behavior, i.e., contractions that pull the tip of the embryonic tail toward the head (Saint-Amant and Drapeau, 1998), was imaged for 1 min durations at 22, 26, 30, and 34 hpf using a Nikon SMZ-1500 stereomicroscope fitted with a Nikon DXM 1200 digital camera (Nikon Instruments Inc., Melville, NY). To quantify coiling frequency, the number of coiling bursts per minute was recorded using Solomon Coder software (ELTE TTK, Budapest, Hungary) for each of the 10 embryos in all replicates of each treatment group. The test was repeated at 26 hpf with THRβ mRNA injected embryos (described below), a time point selected based on the more marked differences observed between treatment groups (Fig. 1). Additionally, the total distance (mm) the tip of tail moved during the course of coiling, the duration (sec) of each coiling burst, and the speed (distance/time) of tip of tail movement during coiling were analyzed for 10 embryos per 6-OH-BDE-47 treatment group in each replicate (n = 3).
Fig. 1. 6-OH-BDE-47 exposure increased coiling frequency.
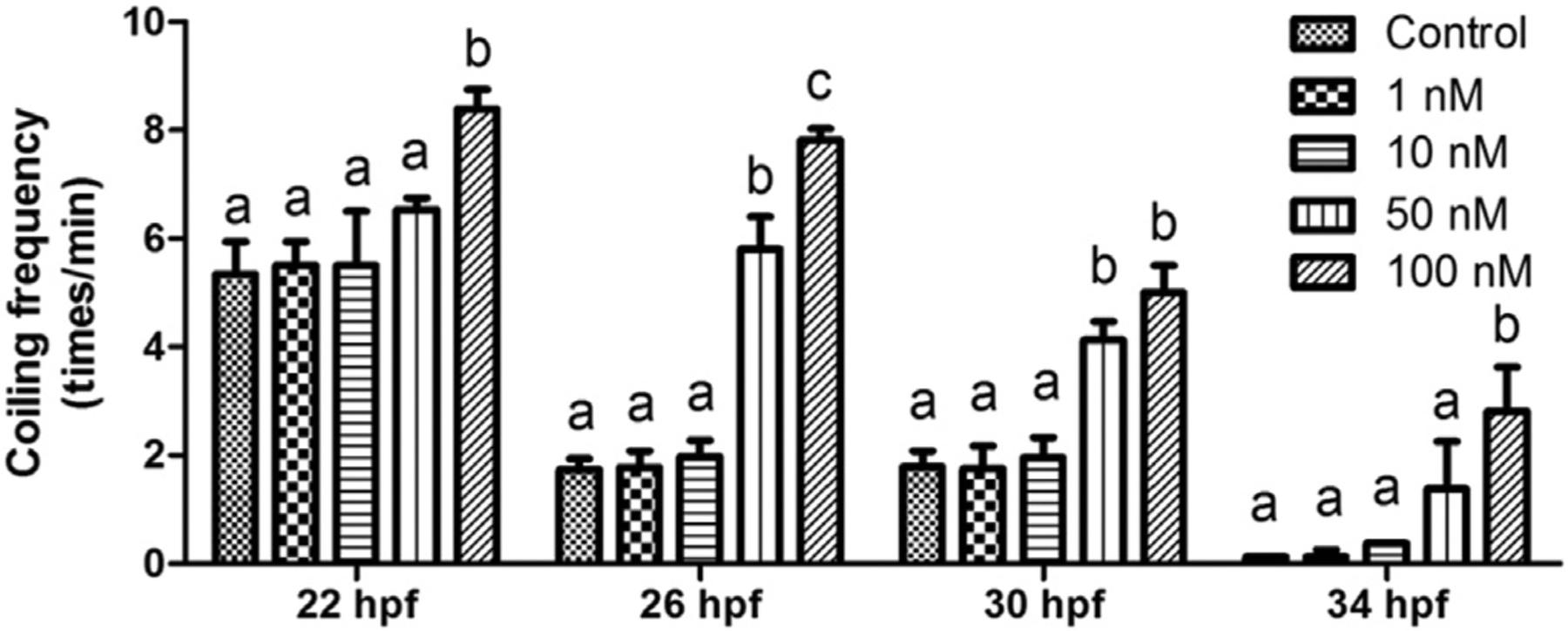
Upon exposure to 0 (control), 1,10, 50 or 100 nM 6-OH-BDE-47 starting at 4h post fertilization (hpf), coiling frequency (times/min) was counted at 22, 26, 30, or 34 hpf from 10 embryos in each of three replicates (n = 3) per treatment group. Different letters indicate significant differences (mean ± SEM; p < 0.05).
2.4. Apoptosis measurement (TUNEL assay)
Ten embryos each from THRβ, 6-OH-BDE-47 (0, 1, 10, 50 and 100 nM), or 6-OH-BDE-47 + THRβ treatment groups were fixed in 4% paraformaldehyde (PFA) overnight, followed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Staining was done on whole mount (26 hpf) and paraffin sectioned (26 hpf) embryos (Dong et al., 2014). Then, a VECTASTAIN Elite ABC HRP kit (PK-6100; Vector Laboratories, Burlingame, CA) and dia-minobenzidine tetrahydrochloride (DAB) solution were used to detect positive TUNEL signals. TUNEL-positive signals in brains were counted under Leica microscope (DM500; Leica Microsystems Inc., Buffalo Grove, IL) equipped with an Olympus DP80 digital microscope camera and Olympus cellSens Standard 1.12 imaging software (Olympus Corporation, Tokyo, Japan).
2.5. THRβ mRNA over expression rescue study
In our previous study, we showed 6-OH-BDE-47 to down-regulate expression of THRβ (Dong et al., 2014). THRβ mRNA was synthesized with SP6 polymerase and capped using a G(5’)ppp(5’)A RNA cap structure analog (New England Biolabs, Ipswich, MA). Embryos were injected with ~3 nL of THRβ (≈265 ng/μL) mRNA in the 1–2 cell stage according to published methods (Macaulay et al., 2015b) using a microinjection system consisting of a Nikon SMZ-1500 stereomicroscope (Nikon Instruments Inc., Melville, NY, USA) and a Narishige IM300 Microinjector (Narishge, Japan). Embryos in control group were injected with ~3 nL of 0.9% NaCl solution. Phenol red (0.05%; Sigma Aldrich) was used to track injections according to Dong et al. (2014).
2.6. Total RNA isolation
A TRIzol™ Plus RNA Purification kit (Grand Island; Thermo Fisher Scientific; 12183–555) was used for RNA extraction of 10 embryos (26 hpf) pooled from each treatment group, following the manufacturer’s instructions. Purity of RNA samples was analyzed by measuring the ratio of absorptions at 260/280 nm using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). cDNA was obtained via reverse transcript from purified RNA samples using High Capacity cDNA Reverse Transcription Kits (Applied Biosystems Inc., Grand Island, NY, USA; Thermo Fisher Scientific; 4368814) in accordance with the reverse transcription protocols of the manufacturer. The cDNA samples were stored at −20 °C until use.
2.7. Real-time PCR
Relative levels of tryptophan hydroxylase 2 (TPH2) and 18s rRNA were measured using real-time PCR. The following real-time PCR primers were designed using PrimerQuest (Integrated DNA Technologies, Coralville, IA, USA): TPH2, forward primer 5′-AGAGGA-CAACATCCCACAGC-3′, reverse primer 5′-CAAGCAGTTCATGGCAGGTG-3′; 18s, forward primer 5′–CCTGCGGCTTAATTTGACTC-3′, reverse primer 5′-GACAAATCGCTCCACCAACT-3′. TPH2 and 18s rRNA were PCR amplified separately in triplicate (three samples from each treatment) using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems) in a 96-well PCR plate. Real-time PCR reaction protocol was 95 °C for 15 min, 41 cycles of 94°C for 15 s, 55 °C for 30 s, and 72 °C for 1 min. Samples were run on the Applied Biosystems 7900 H T instrument using their Sequence Detection System 2.0 software. 18s rRNA was used to normalize target gene expression, in order to compensate for intrinsic variability in the amount of RNA between embryos, and threshold cycle (Ct) was normalized with 18s rRNA of the same sample according to a previous method (Chen et al., 2004). The amplification was calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
2.8. 5-HT neuronal antibody staining
Ten larvae in each treatment group were fixed in 4% PFA at 96 hpf, a time at which the hypothalamus is developed. Paraffin sections of eleutheroembryos in parasagittal plane were prepared according to our previous methods (Dong et al., 2014). Sections were treated with proteinase K (Gibco, Carlsbad, USA) in 1 × PBS at 37 °C for 10 min and then washed with PBS containing 0.1% Tween-20 (PBST). After blocking with 2% bovine serum albumin (BSA) in PBST, embryos were incubated overnight with 1:500 rabbit polyclonal 5-HT antibody (ImmunoStar, Hudson, USA). After multiple washes in PBST, slides were incubated in 2% PBST containing 1:200 biotin-labeled goat anti-rabbit secondary antibody (Beyotime, Jiangsu, China) for 2 h at room temperature, followed by 1 h incubation with A-B solution from the VECTASTATIN Elite kit (Vector Laboratories, Ltd.). Finally, color reaction was performed with DAB solution. At this time, 5-HT-ir neurons were localized to hypothalamus and were counted for each individual using a Leica DM500 microscope fitted Olympus DP80 digital camera.
2.9. Statistical analyses
Data were expressed as mean ± SEM and analyzed with GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). The differences between means were analyzed using oneway ANOVA followed by Tukey’s post-hoc test. Statistical significance among groups was considered to be p < 0.05. Correlation coefficients were also analyzed with GraphPad Prism software.
3. Results
3.1. 6-OH-BDE-47 exposure increased coiling frequency
Coiling frequency at 22, 26, 30, and 34 hpf in 6-OH-BDE-47 exposed zebrafish embryos is shown in Fig. 1. At 22 hpf, control embryos coiled 5.4 times/min. Only the 100 nM group had a significantly higher coiling frequency (p = 0.0482), with 8.4 times/min. At 26 hpf, control embryos coiled 1.7 times/min. The 50 and 100 nM groups had a significantly higher coiling frequency (5.9 and 7.8 times/min, respectively), with 100 nM exposed embryos coiling the most frequently (p = 0.0358). At 30 hpf, control embryos coiled 1.8 times/min. The 50 and 100 nM groups had a significantly higher coiling frequency (4.1 and 5.0 times/min, p = 0.0351 and 0.0310, respectively). At 34 hpf, the coiling frequency of all embryos was reduced greatly compared with the corresponding concentration group at earlier stages. Control embryos coiled 0.125 times/min. Only 100 nM group had a significant higher coiling frequency (2.8 times/min) compared to the control group (p = 0.0045).
To exclude the possible involvement of muscular function in the effects of 6-OH-BDE-47, we used software to analyze coiling behavior in zebrafish embryo at 26 hpf. We measured three indices: the total distance the tip of tail moved during the course of the coiling behavior, the time (sec) of each coiling behavior, and the speed (distance/time) of tip of tail movement during each coiling behavior. There were no significant differences among each 6-OH-BDE-47 treatment group for any of these indices (Fig. S1).
3.2. Rescue of coiling frequency with THRβ mRNA overexpression
THRβ mRNA overexpression was used to rescue the adverse effects of 6-OH-BDE-47 in zebrafish embryos. Groups included embryos exposed to 6-OH-BDE-47 (1,10, 50,100 nM) with (+/+) or without THRβ mRNA injections (±) (Fig. 2). At 26 hpf, there was no significant difference between coiling frequency of negative control embryos (1.8 times/min) and those injected only with THRβ mRNA (2.0 times/min). However, the 50 and 100 nM 6-OH-BDE-47 groups had a significantly higher coiling frequency (3.4 and 4.6 times/min, respectively) relative to control group. THRβ mRNA injections rescued the 50 nM 6-OH-BDE-47 embryos and partially rescued 100 nM 6-OH-BDE-47 exposed embryos.
Fig. 2. THRβ mRNA overexpression rescued increased coiling frequency.
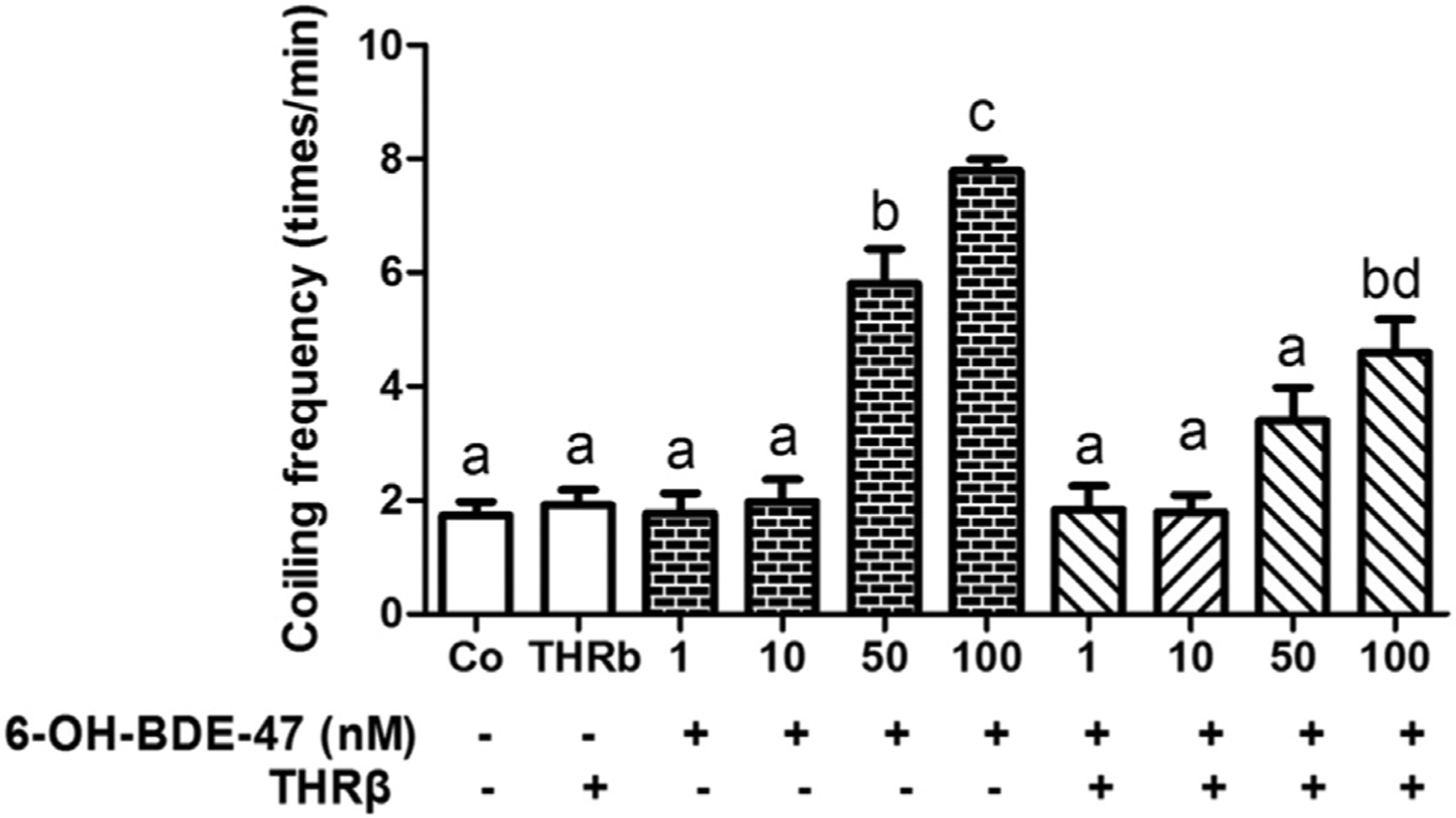
Embryos, with (+) and without (−) injection of THRβ mRNA at the 1–2 cell stage, were exposed to 0 (control), 1,10, 50 or 100 nM 6-OH-BDE-47 from 4 to 26 hpf. At 26 hpf, coiling frequency (times/min) was counted from 10 embryos in three replicates (n = 3) per treatment group. Different letters indicate significant differences (mean ± SEM; p < 0.05).
3.3. THRβ mRNA overexpression rescued 6-OH-BDE-47 induced apoptosis in the brain
To localize the position of apoptotic cells in the brains of zebrafish embryos at 26 hpf, we carried out the TUNEL staining on tissue of sectioned embryos. Few apoptotic cells were observed in brains of controls (Fig. 3A), indicating a low background level of apoptosis in the brain at this developmental stage. In the THRβ mRNA control group, the number of TUNEL-positive cells (i.e., apoptotic cells, brown colored in the figure) was also low (Fig. 3F). In contrast, an increase in the number of apoptotic cells in the brain was detected in the 1, 10, 50 and 100 nM 6-OH-BDE-47 embryos (Fig. 3B–E). Interestingly, overexpression of THRβ mRNA rescued this effect, as evidenced by the low number of apoptotic cells, a level comparable that of the controls (Fig. 3G–J).
Fig. 3. THRβ mRNA overexpression rescued 6-OH-BDE-47 induced apoptosis in brain sections of embryos.
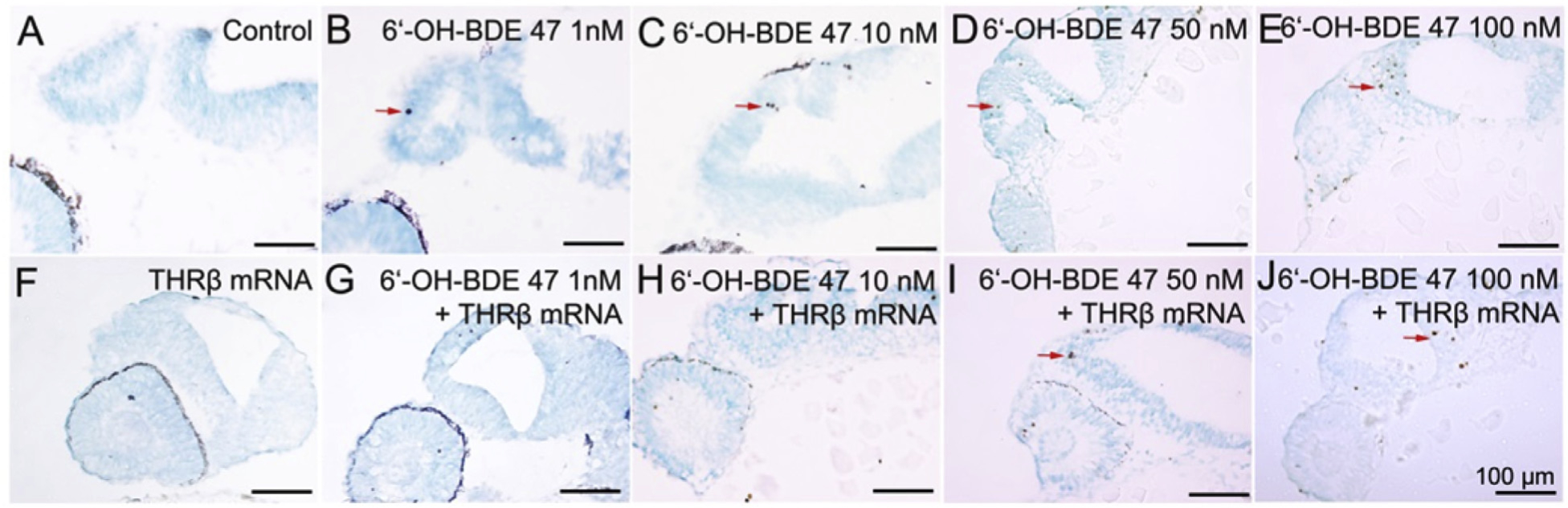
Embryos, with and without injection of THRβ mRNA at the 1–2 cell stage, were exposed to 0 (control), 1,10, 50 or 100 nM 6-OH-BDE-47 from 4 to 26 hpf. At 26 hpf, embryos were sectioned and TUNEL stained. TUNEL-positive, brown colored cells (i.e., apoptotic cells), were in the brain (red arrows) where they increased in a concentration-dependent manner after 6-OH-BDE-47 exposure (A–E). This effect was rescued by overexpression of THRβ mRNA (F–J). All images are at the same magnification, scale bars are 100 mm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
To further quantify apoptotic cells, whole mount TUNEL staining in 26 hpf zebrafish embryos was conducted and the number of TUNEL positive cells counted. There were 2, 2, 2,16,19, 28, 2,10,12, and 18 apoptotic cells/embryo in 0 (control), 1,10, 50, and 100 nM 6-OH-BDE-47, THRβ, 1 nM + THRβ, 10 nM + THRβ, 50 nM + THRβ, and 100 nM + THRβ treatment groups, respectively (Fig. 4A–F). Compared to the control, 10 nM 6-OH-BDE-47 exposed embryos had 8 times more apoptotic cells (p = 0.0007), 50 nM 6-OH-BDE-47 exposed embryos had 9 times more apoptotic cells (p < 0.0001), and 100 nM 6-OH-BDE-47 exposed embryos had 14 times more apoptotic cells (p < 0.0001) (Fig. 4G). THRβ mRNA injection resulted in a significant recovery of 6-OH-BDE-47 exposed embryos, reducing the number of apoptotic cells two fold compared with 6-OH-BDE-47 treatment alone (Fig. 4K).
Fig. 4. THRβ mRNA overexpression rescued 6-OH-BDE-47 induced apoptosis in the brain of whole mount zebrafish embryos.
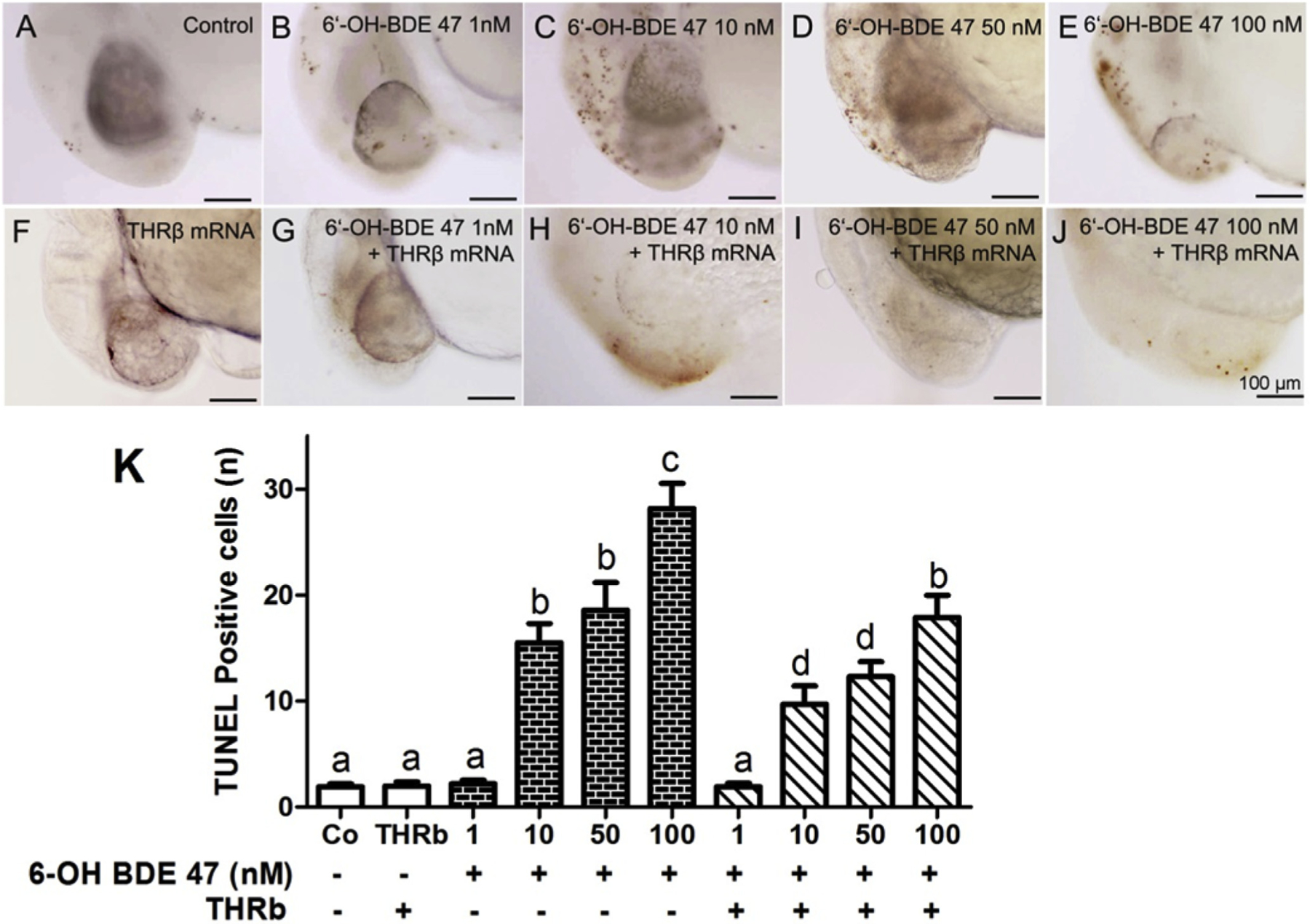
Embryos, with and without injection ofTHRβ mRNA at the 1–2 cell stage, were exposed to 0 (control), 1, 10, 50 or 100 nM 6-OH-BDE-47 from 4 to 26 hpf. TUNEL-positive, brown colored cells (i.e., apoptotic cells) in the brain increased in a concentration-dependent manner in 6-OH-BDE-47 exposed embryos (A–E). This effect was rescued by overexpression ofTHRβ mRNA (F–J). Mean ± SEM of discrete TUNEL positive reactions of 10 embryos in three replicates (n = 3) per treatment group are shown in histogram (K). Different letters indicate significant differences (p < 0.05). All images are at the same magnification, scale bars are 100 mm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Increased coiling frequency correlated with increased apoptosis in brains of 6-OH-BDE-47 exposed embryos
To determine if there was a correlation between increased coiling frequency and increased apoptosis in the brain resulting from 6-OH-BDE-47 exposure, a correlation coefficient analysis was conducted. Results showed a strongly positive linear relationship between the numbers of apoptotic cells and coiling frequency (r = 0.8788, p = 0.0497) (Fig. 5).
Fig. 5. Increased coiling frequency and apoptotic cells are correlated in brains of 6-OH-BDE-47 exposed embryos.
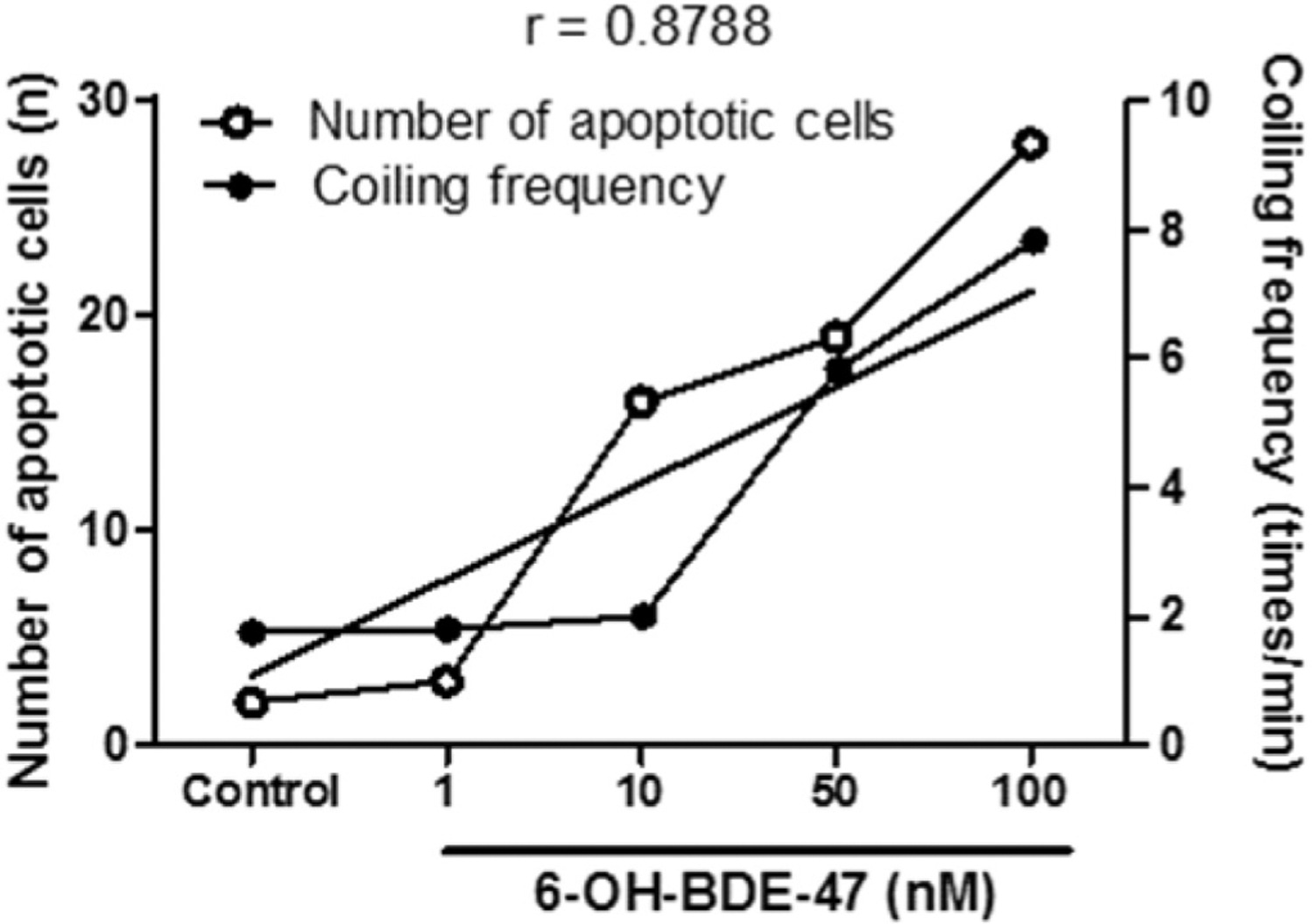
Zebrafish embryos were exposed to 0 (control), 1,10, 50, or 100 nM 6-OH-BDE-47 from 4 to 26 hpf. Coiling frequency and the number of apoptotic cells were measured, and a correlation coefficient calculated. These measurements are strongly, positively correlated (r = 0.8788; p = 0.0497).
3.5. 6-OH-BDE-47 down-regulated TPH2 mRNA expression
At 26 hpf, no significant changes in the TPH2 mRNA expression were found in the 1,10, and 50 nM 6-OH-BDE-47 treatment groups compared to control. However, embryos exposed to 100 nM had a significant decrease (16.9%; p = 0.0003) of TPH2 mRNA expression (Fig. 6).
Fig. 6. 6-OH-BDE-47 down-regulates TPH2 mRNA expression in zebrafish embryos.
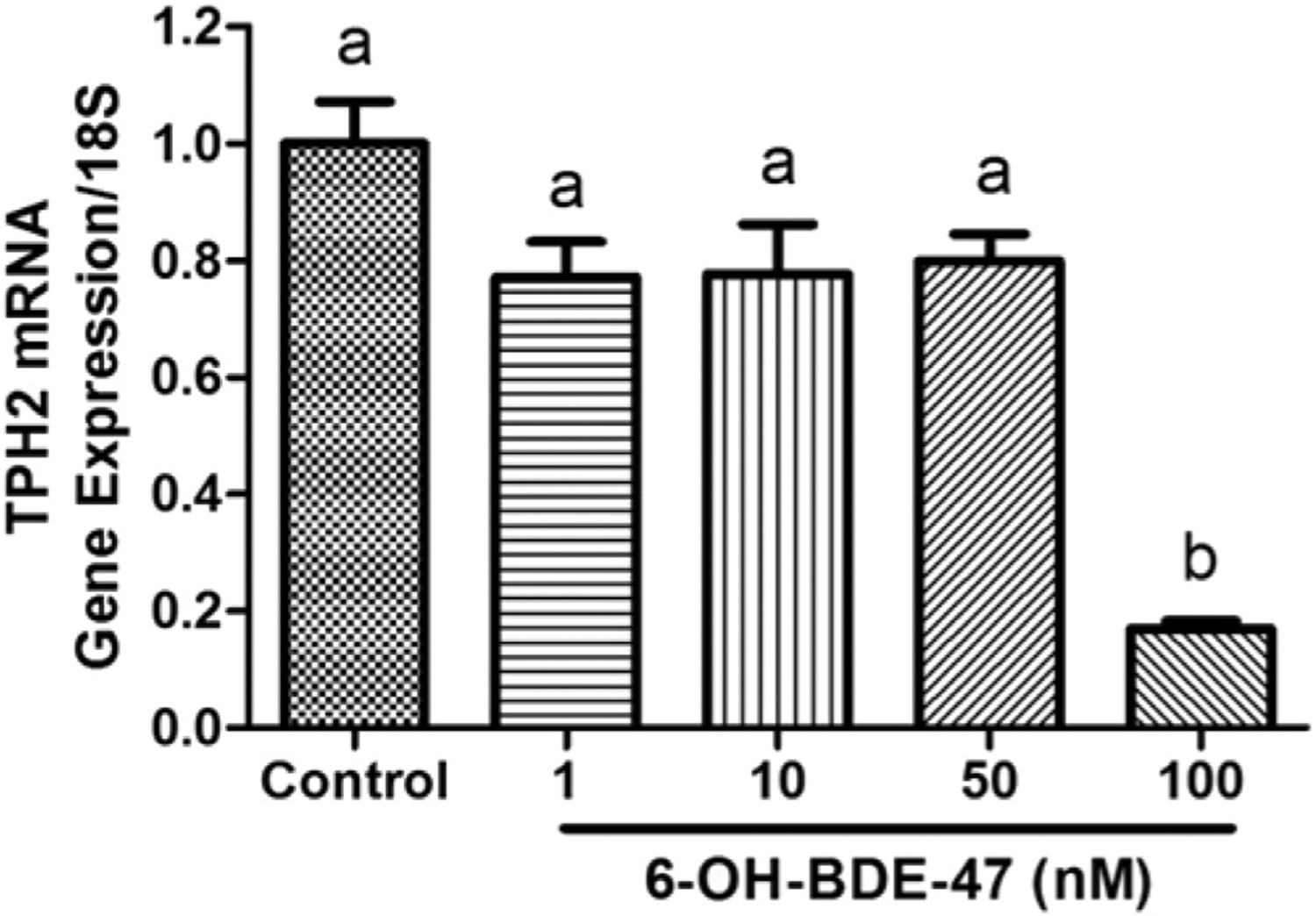
TPH2 mRNA gene expression at 26 hpf in zebrafish embryos exposed to 0 (control), 1, 10, 50, or 100 nM 6-OH-BDE-47. Each group contained 10 embryos in three replicates (n = 3) per treatment group. Different letters indicate significant differences (mean ± SEM; p < 0.05).
3.6. 6-OH-BDE-47 reduced 5-HT-ir neurons in zebrafish hypothalamus
To localize and quantify 5-HT-ir neurons, we carried out antibody staining on 96-hpf zebrafish larvae that were paraffin sectioned and compared anatomy using the PennState Bio-Atlas (http://bio-atlas.psu.edu/). 5-HT positive (i.e., brown colored) neurons were found in the caudal ventricular recess of hypothalamus (Fig. 7). Control larvae had a mean of 30 5-HT-ir neurons (Fig. 7A,F). The number of 5-HT-ir neurons was 30, 22,15, and 13 in the larvae from the 1, 10, 50, and 100 nM 6-OH-BDE-47 treatment groups, respectively, showing a concentration-dependent response. The 50 nM treatment group had 43% fewer 5-HT-ir neurons and 100 nMgroups 50% fewer than the control (p = 0.0013 and 0.0030, respectively; Fig. 7D–E, J–K).
Fig. 7. 6-OH-BDE-47 reduces 5-HT-ir neurons in zebrafish hypothalamus.
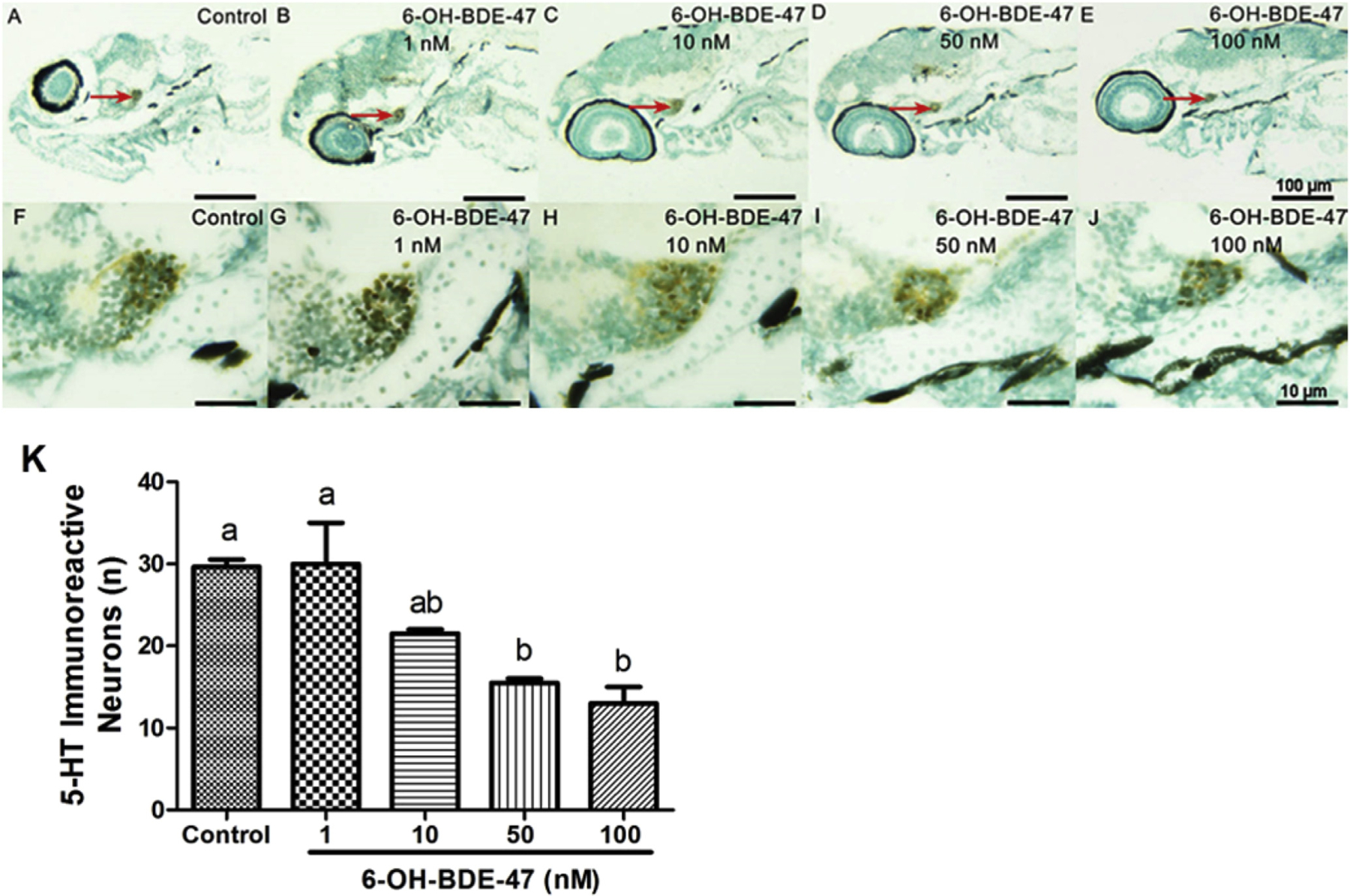
5-HT-ir neurons in 96-hpf zebrafish larvae exposed to 0 (control), 1,10, 50, or 100 nM 6-OH-BDE-47 from 4 to 96 hpf. 5-HT-ir neurons (brown colored) were found in the caudal ventricular recess of hypothalamus (A–J). A–E: 5-HT-ir neurons in hypothalamus (red arrows); F–J: magnifications of A-E showing 5-HT-ir neurons in hypothalamus. Histogram (K) shows counts of 5-HT-ir neurons in hypothalamus. Bars are means ± SEM of 10 individual larvae per treatment. Different letters indicate significant differences (p < 0.05). A-E are at the same magnification, scale bars are 100 mm; F-J are at the same magnification, scale bars are 10 mm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
We used alterations in coiling behavior to evaluate neurological toxicity in early developmental stages of zebrafish embryos. Our results demonstrated that 6-OH-BDE-47 exposure led to increased coiling frequency, decreased TPH2 gene expression, increased apoptosis in the brain, and reduced the number of 5-HT-ir neurons in the hypothalamus. Importantly, increased coiling frequency and apoptosis in the brain were partially rescued by THRβ mRNA overexpression. It is likely that 6-OH-BDE-47 affected TH regulation through THRβ, which in turn affected neurological behavior as evidenced by changes in coiling frequency.
Spontaneous movement begins at 17 hpf as evidenced by slow and alternating tail coiling. Starting at 21 hpf, embryos coil their tails in response to stimuli (Saint-Amant and Drapeau, 1998; McKeown et al., 2009). We observed overall decreased coiling frequency as control embryos developed. However, embryos exposed to 6-OH-BDE-47 showed a concentration-dependent increase in the frequency of coiling at each of the developmental time points evaluated. We postulated that this effect could be described as delayed development. Our previous study using the same concentrations of 6-OH-BDE-47 and examining eye area supported increased development, i.e., no delay (Dong et al., 2014). Therefore, more studies will be needed to classify the alterations as developmental delay.
It is also possible that increased coiling frequency could have resulted from effects of 6-OH-BDE-47 on muscular function. If 6-OH-BDE-47 affected muscular function, we would expect alterations in movement (e.g., muscle twitch) relative to controls (Baylor and Hollingworth, 2012). However, there were no significant differences between any groups for the three indices measured. This led to our conclusion that increased coiling frequency was related to neurotoxicity of 6-OH-BDE-47.
Apoptosis of brain cells is an important neurotoxicity phenotype (Sheng et al., 2016). In the zebrafish brain stem, Mauthner cells, a type of reticulospinal (RS) neuron, form synaptic contacts with trunk motor neurons and can initiate the C-shaped turning, a fast escape reflex. Damage to neurons of the brain could alter hormone production pathways and eventually affect the coiling behavior (Jontes et al., 2000; Umeda and Shoji, 2017). In the present study, 6-OH-BDE-47 exposed embryos had significantly more apoptotic cells compared to controls. This apoptosis was concentration-dependent and widespread in the brain, likely also in the brain stem. Furthermore, this apoptosis was strongly, positively correlated with increased coiling frequency, suggesting the two responses are related. PBDEs have been shown to promote similar spontaneous movements in other animals. For instance, mice exposed to PBDEs displayed a significant hyperactivity over controls after a 60-min exposure (Eriksson et al., 2001; Bowers et al., 2015). Zebrafish larvae exposed to DE-71 until 15 days post fertilization (dpf) had increased optokinetic responses, which depend on a functional retina and optic nerve and are mediated by different parts of the brain (Chen et al., 2013b). Additionally, these larvae showed an increase in light-seeking behavior that may have a neurobiogical basis (Chen et al., 2013b). Conversely, neurotoxicity studies have demonstrated that exposure to inorganic mercury, ethanol, or the herbicide, diuron inhibited coiling behavior in zebrafish embryos at 24 hpf (Abu Bakar et al., 2017; Ramlan et al., 2017; Velki et al., 2017). These authors suggested this inhibition might be linked to damage or decreased differentiation of neurons in the spinal cord and neural tube. In addition, Macaulay et al. (2015a) showed that zebrafish exposed to 10–50 nM 6-OH-BDE-47 during embryonic and larval development (0–6 dpf) had some behavioral deficits or alterations, including swimming activity, fear response, novel environment exploration, and habituation learning. Both promotion or inhibition of coiling behavior could be an indicator of altered neurological function.
In addition to coiling behavior and apoptosis, exposure to 6-OH-BDE-47 significantly decreased THRβ mRNA expression in zebrafish embryos (Zheng et al., 2012; Dong et al., 2014). In our study, THRβ mRNA overexpression was performed to rescue the neurological damage (i.e., increased coiling frequency and apoptotic cells in the brain). In the 50 and 100 nM 6-OH-BDE-47 treatment groups, injection of THRβ significantly rescued the embryos from these adverse effects. Not only did THRβ overexpression partially rescue these two effects, it also strengthens our hypothesis that increased coiling frequency and apoptosis of brain cells are linked. However, additional work is needed to pinpoint the exact cell types affected. A similar correlation was demonstrated in zebrafish embryos with an antisense knockdown of cables1 that led to both an increase in apoptotic cells in brain tissue and hyperactivity in response to stimulation (Groeneweg et al., 2011). Apoptosis in the brain during early life stages could also be related to other phenotypes later in life including reductions in swimming behavior, eye movement, impaired cognitive ability and recognition memory (Dou and Zhang, 2011; Shih et al., 2013; Guo et al., 2015; Sheng et al., 2016).
There are two possible explanations to account for the neurotoxicity of 6-OH-BDE-47 (Fig S3). The first is down-regulation of THRβ mRNA by 6-OH-BDE-47. THRβ is a transcription factor mediating the pleiotropic activities of THs (Jones et al., 2003; Rivas and Naranjo, 2007). In the brain and sensory systems, THRβ mediates both hormone-dependent and hormone-independent actions with changing THs levels during development (Jones et al., 2003). THRβ deficiency has been shown to cause dysfunction of the monoaminergic system in mice (Ookubo et al., 2015). In our study, THRβ mRNA overexpression partially rescued the increased apoptosis of brain cells, implying it has an important role in the brain. Another explanation relates to the reduction of 5-HT-ir neurons by 6-OH-BDE-47 observed in this study. The role of 5-HT in the induction of apoptosis (Trouche et al., 2010; Liu et al., 2013), may be important, especially since 5-HT depletion reduces 5-HT-ir neurons, resulting in neurotoxicity and behavioral disorders (Musumeci et al., 2015; Horzmann and Freeman, 2016; Wang et al., 2016; Schoofs et al., 2017). Adult zebrafish exposed to polycyclic aromatic hydrocarbons (PAHs) were depleted of 5-HT and paralleling monoamine concentrations, and they exhibited increased anxiety and disrupted memory regulation and learning (Vignet et al., 2017). In serotonin transporter (5-HTT) knockout mice, excess serotonin disrupted the normal wiring of the somatosensory cortex (Persico et al., 2001) and altered the neuronal migration, contributing to subtle changes in the thickness of layers observed in different cortical regions (Altamura et al., 2007). It is probable that 5-HT-ir neurons have a role in the neurotoxicity evident in our study.
5. Conclusions
6-OH-BDE-47 increased coiling frequency in early stage zebrafish embryos. This behavioral change was linked to 6-OH-BDE-47 induced apoptosis in the brains. 6-OH-BDE-47 exposure also reduced TPH2 expression in embryos and 5-HT-ir neurons in larvae. THRβ mRNA overexpression partially rescued the above as seen by reduction of apoptosis to control levels and decreased coiling frequency, suggesting that it was 6-OH-BDE-47 that disrupted thyroid hormones. Taken together, coiling behavior could potentially be used as an in vivo method to investigate neurological toxicity in early stage embryos.
Supplementary Material
HIGHLIGHTS.
6-OH-BDE-47 resulted in a concentration-dependent increase in coiling frequency.
6-OH-BDE-47 exposure increased apoptosis in brains of embryos.
THRβ mRNA overexpression partially rescued adverse effects.
6-OH-BDE-47 reduced 5-HT-ir neurons and TPH2 expression.
Coiling frequency could be used as an indicator of neurotoxicity in embryos.
Acknowledgments
This work was funded by: National Natural Science Foundation of China (81360508, 21267015, 21567019); Natural Science Foundation of Inner Mongolia Autonomous Region of China (Nos. 2015MS0804); Inner Mongolia Innovation program (KJCX15003); Interdisciplinary Program for Inner Mongolia University for Nationalities (MDXK008); Open Project Program of Inner Mongolia Key Laboratory of Toxicant Monitoring and Toxicology (MDK2017012, MDK2017014); National Institute of Environmental Health Sciences (NIEHS) Duke University Superfund Research Center (P42ES010356).
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.chemosphere.2018.01.081.
References
- Abu Bakar N, Mohd Sata NS, Ramlan NF, Wan Ibrahim WN, Zulkifli SZ, Che Abdullah CA, Ahmad S, Amal MN, 2017. Evaluation of the neurotoxic effects of chronic embryonic exposure with inorganic mercury on motor and anxietylike responses in zebrafish (Danio rerio) larvae. Neurotoxicol. Teratol 59,53–61. [DOI] [PubMed] [Google Scholar]
- Altamura C, Dell’Acqua ML, Moessner R, Murphy DL, Lesch KP, Persico AM, 2007. Altered neocortical cell density and layer thickness in serotonin transporter knockout mice: a quantitation study. Cerebr. Cortex 17, 1394–1401. [DOI] [PubMed] [Google Scholar]
- Asan E, Steinke M, Lesch KP, 2013. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem. Cell Biol 139, 785–813. [DOI] [PubMed] [Google Scholar]
- Bailey J, Oliveri A, Levin ED, 2013. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res C Embryo Today 99,14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S, 2012. Intracellular calcium movements during excitation-contraction coupling in mammalian slow-twitch and fast-twitch muscle fibers. J. Gen. Physiol 139, 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers WJ, Wall PM, Nakai JS, Yagminas A, Wade M, Li N, 2015. Behavioral and thyroid effects of in utero and lactational exposure of Sprague-Dawley rats to the polybrominated diphenyl ether mixture DE71. Neurotoxicol. Teratol 52, 127–142. [DOI] [PubMed] [Google Scholar]
- Brustein E, Chong M, Holmqvist B, Drapeau P, 2003. Serotonin patterns locomotor network activity in the developing zebrafish by modulating quiescent periods. J. Neurobiol 57, 303–322. [DOI] [PubMed] [Google Scholar]
- Butt CM, Stapleton HM, 2013. Inhibition of thyroid hormone sulfotransferase activity by brominated flame retardants and halogenated phenolics. Chem. Res. Toxicol 26, 1692–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Wang D, Stapleton HM, 2011. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol. Sci 124, 339–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Park JS, Linderholm L, Rhee A, Petreas M, DeFranco EA, Dietrich KN, Ho SM, 2013a. Hydroxylated polybrominated diphenyl ethers in paired maternal and cord sera. Environ. Sci. Technol 47, 3902–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Tang X, Zhou B, Xu N, Zhou Z, Fang K, Wang Y, 2017. BDE-47 and BDE-209 inhibit proliferation of Neuro-2a cells via inducing G1-phase arrest. Environ. Toxicol. Pharmacol 50, 76–82. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang Y, Huang C, Hu B, Hu C, Zhou B, 2013b. Acute exposure to DE-71 causes alterations in visual behavior in zebrafish larvae. Environ. Toxicol. Chem 32, 1370–1375. [DOI] [PubMed] [Google Scholar]
- Chen W, John J, Lin C, Lin H, Wu S, Lin C, Chang C, 2004. Expression of metallothionein gene during embryonic and early larval development in zebrafish. Aquat. Toxicol 69, 215–227. [DOI] [PubMed] [Google Scholar]
- Covaci A, Voorspoels S, de Boer J, 2003. Determination of brominated flame retardants, with emphasis on polybrominated diphenyl ethers (PBDEs) in environmental and human samples–a review. Environ. Int 29, 735–756. [DOI] [PubMed] [Google Scholar]
- Cowell WJ, Lederman SA, Sjodin A, Jones R, Wang S, Perera FP, Wang R, Rauh VA, Herbstman JB, 2015. Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3–7 years. Neurotoxicol. Teratol 52, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M, 2001. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ. Health Perspect 109 (Suppl. 1), 49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, de Groot A, van Kleef RG, Bergman A, van den Berg M, Vijverberg HP, Westerink RH, 2008. Hydroxylation increases the neurotoxic potential of BDE-47 to affect exocytosis and calcium homeostasis in PC12 cells. Environ. Health Perspect 116, 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, Ramakers GM, Gardoni F, van Kleef RG, Bergman A, Di Luca M, van den Berg M, Westerink RH, Vijverberg HP, 2007. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ. Health Perspect 115, 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Macaulay LJ, Kwok KW, Hinton DE, Ferguson PL, Stapleton HM, 2014. The PBDE metabolite 6-OH-BDE 47 affects melanin pigmentation and THRbeta MRNA expression in the eye of zebrafish embryos. Endocr. Disruptors (Austin) 2, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou C, Zhang J, 2011. Effects of lead on neurogenesis during zebrafish embryonic brain development. J. Hazard Mater 194, 277–282. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A, 2001. Brominated flame retardants a novel class of developmental neurotoxicants. Environ. Health Perspect 109, 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L, 2003. The developmental role ofserotonin: news from mouse molecular genetics. Nat. Rev. Neurosci 4, 1002–1012. [DOI] [PubMed] [Google Scholar]
- Gee J, Moser V, 2008. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol. Teratol 30, 79–87. [DOI] [PubMed] [Google Scholar]
- Groeneweg JW, White YA, Kokel D, Peterson RT, Zukerberg LR, Berin I, Rueda BR, Wood AW, 2011. Cables1 is required for embryonic neural development: molecular, cellular, and behavioral evidence from the zebrafish. Mol. Reprod. Dev 78, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Huang Z, Tao T, Chen X, Zhang W, Zhang Y, Lin C, 2015. Zebrafish as a model for studying the developmental neurotoxicity of propofol. J. Appl. Toxicol 35, 1511–1519. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJ, Brouwer A, Bergman A, 2008. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2’,4,4’-tetrabromodiphenyl ether (BDE-47). Mol. Nutr. Food Res 52, 284–298. [DOI] [PubMed] [Google Scholar]
- He P, He W, Wang A, Xia T, Xu B, Zhang M, Chen X, 2008. PBDE-47-induced oxidative stress, DNA damage and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology 29, 124–129. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES, 2003. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxietylike and aggressive behavior. Neuron 37, 233–24. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Mall JK, 2014. Developmental exposure to polybrominated diphenyl ethers and neurodevelopment. Curr. Environ. Health Rep 1, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F, 2010. Prenatal exposure to PBDEs and neurodevelopment. Environ. Health Perspect 118, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA, 2004. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ. Sci. Technol 38, 945–956. [DOI] [PubMed] [Google Scholar]
- Horzmann KA, Freeman JL, 2016. Zebrafish get connected: investigating neurotransmission targets and alterations in chemical toxicity. Toxics 4, 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones I, Srinivas M, Ng L, Forrest D, 2003. The thyroid hormone receptor beta gene: structure and functions in the brain and sensory systems. Thyroid 13, 1057–1068. [DOI] [PubMed] [Google Scholar]
- Jontes JD, Buchanan J, Smith SJ, 2000. Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat. Neurosci 3, 231–23. [DOI] [PubMed] [Google Scholar]
- Legradi J, Dahlberg AK, Cenijn P, Marsh G, Asplund L, Bergman A, Legler J, 2014. Disruption of oxidative phosphorylation (OXPHOS) by hydroxylated polybrominated diphenyl ethers (OH-PBDEs) present in the marine environment. Environ. Sci. Technol 48, 14703–14711. [DOI] [PubMed] [Google Scholar]
- Liu D, Wu SM, Zhang Q, Guo M, Cheng J, Zhang SH, Yao C, Chen JQ, 2017. Occurrence, spatial distribution, and ecological risks of typical hydroxylated polybrominated diphenyl ethers in surface sediments from a large freshwater lake of China. Environ. Sci. Pollut. Res. Int 24, 5773–5780. [DOI] [PubMed] [Google Scholar]
- Liu H, Tang S, Zheng X, Zhu Y, Ma Z, Liu C, Hecker M, Saunders DM, Giesy JP, Zhang X, Yu H, 2015. Bioaccumulation, biotransformation, and toxicity of BDE-47, 6-OH-BDE-47, and 6-MeO-BDE-47 in early life-stages of zebrafish (Danio rerio). Environ. Sci. Technol 49, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tian HY, Yan XL, Fan FL, Wang WP, Han JL, Zhang JB, Ma Q, Meng Y, Wei F, 2013. Serotonin inhibits apoptosis of pulmonary artery smooth muscle cell by pERK1/2 and PDK through 5-HT1B receptors and 5-HT transporters. Cardiovas Pathol. Off. J. Soc. Cardiovas. Pathol 22, 451–457. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Macaulay LJ, Bailey JM, Levin ED, Stapleton HM, 2015a. Persisting effects of a PBDE metabolite, 6-OH-BDE-47, on larval and juvenile zebrafish swimming behavior. Neurotoxicol. Teratol 52, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay LJ, Chen A, Rock KD, Dishaw LV, Dong W, Hinton DE, Stapleton HM, 2015b. Developmental toxicity of the PBDE metabolite 6-OH-BDE-47 in zebrafish and the potential role of thyroid receptor beta. Aquat. Toxicol 168, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown KA, Downes GB, Hutson LD, 2009. Modular laboratory exercises to analyze the development of zebrafish motor behavior. Zebrafish 6, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci G, Castrogiovanni P, Castorina S, Imbesi R, Szychlinska MA, Scuderi S, Loreto C, Giunta S, 2015. Changes in serotonin (5-HT) and brain-derived neurotrophic factor (BDFN) expression in frontal cortex and hippocampus of aged rat treated with high tryptophan diet. Brain Res. Bull 119, 12–18. [DOI] [PubMed] [Google Scholar]
- Ookubo M, Sadamatsu M, Yoshimura A, Suzuki S, Kato N, Kojima H, Yamada N, Kanai H, 2015. Aberrant monoaminergic system in thyroid hormone receptor-β deficient mice as a model of attention-deficit/hyperactivity disorder. Int. J. Neuropsychopharmacol 18, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla S, Hunter DL, Padnos B, Frady S, MacPhail RC, 2011. Assessing locomotor activity in larval zebrafish: influence of extrinsic and intrinsic variables. Neurotoxicol. Teratol 33, 624–630. [DOI] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F, 2001. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J. Neurosci 21, 6862–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlan NF, Sata N, Hassan SN, Bakar NA, Ahmad S, Zulkifli SZ, Abdullah CAC, Ibrahim WNW, 2017. Time dependent effect of chronic embryonic exposure to ethanol on zebrafish: morphology, biochemical and anxiety alterations. Behav. Brain Res 332, 40–49. [DOI] [PubMed] [Google Scholar]
- Ren XMG, Guo Liang-Hong, 2013. Molecular toxicology of polybrominated diphenyl ethers: nuclear hormone receptor mediated pathways. Environ. Sci. Process Impacts 15, 702–708. [DOI] [PubMed] [Google Scholar]
- Rivas M, Naranjo JR, 2007. Thyroid hormones, learning and memory. Gene Brain Behav. 6 (Suppl. 1), 40–44. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P, 1998. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol 37, 622–632. [DOI] [PubMed] [Google Scholar]
- Schoofs A, Huckesfeld S, Pankratz MJ, 2017. Serotonergic network in the sub-esophageal zone modulates the motor pattern for food intake in Drosophila. J. Insect Physiol. 10.1016/j.jinsphys.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Gassmann K, Gotz C, Hubenthal U, Moors M, Krause G, Merk HF, Nguyen NH, Scanlan TS, Abel J, Rose CR, Fritsche E, 2010. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ. Health Perspect 118, 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L, Wang L, Su M, Zhao X, Hu R, Yu X, Hong J, Liu D, Xu B, Zhu Y, Wang H, Hong F, 2016. Mechanism of TiO2 nanoparticle-induced neurotoxicity in zebrafish (Danio rerio). Environ. Toxicol 31, 163–175. [DOI] [PubMed] [Google Scholar]
- Shih DF, Hsiao CD, Min MY, Lai WS, Yang CW, Lee WT, Lee SJ, 2013. Aromatic L-amino acid decarboxylase (AADC) is crucial for brain development and motor functions. PLoS One 8, e7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG Jr., 2008. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Sci. Technol 42, 1377–1384. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML, 2011. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ. Health Perspect 119, 1454–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridi A, Tsalafouta A, Pavlidis M, 2017. Acute exposure to fluoxetine alters aggressive behavior of zebrafish and expression of genes involved in serotonergic system regulation. Front. Neurosci 11, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche E, Mias C, Seguelas M-H, Ordener C, Cussac D, Parini A, 2010. Characterization of monoamine oxidases in mesenchymal stem cells: role in hydrogen peroxide generation and serotonin-dependent apoptosis. Stem Cell. Dev 19, 1571+. [DOI] [PubMed] [Google Scholar]
- Umeda K, Shoji W, 2017. From neuron to behavior: sensory-motor coordination of zebrafish turning behavior. Dev. Growth Differ 59, 107–114. [DOI] [PubMed] [Google Scholar]
- Usenko CY, Hopkins DC, Trumble SJ, Bruce ED, 2012. Hydroxylated PBDEs induce developmental arrest in zebrafish. Toxicol. Appl. Pharmacol 262, 43–51. [DOI] [PubMed] [Google Scholar]
- Usenko CY, Robinson EM, Usenko S, Brooks BW, Bruce ED, 2011. PBDE developmental effects on embryonic zebrafish. Environ. Toxicol. Chem 30, 1865–1872. [DOI] [PubMed] [Google Scholar]
- Valters K, Li H, Alaee M, D’Sa I, Marsh G, Bergman A, Letcher RJ, 2005. Polybrominated diphenyl ethers and hydroxylated and methoxylated brominated and chlorinated analogues in the plasma of fish from the Detroit River. Environ. Sci. Technol 39, 5612–5619. [DOI] [PubMed] [Google Scholar]
- van Boxtel AL, Kamstra JH, Cenijn PH, Pieterse B, Wagner JM, Antink M, Krab K, van der Burg B, Marsh G, Brouwer A, Legler J, 2008. Microarray analysis reveals a mechanism of phenolic polybrominated diphenylether toxicity in zebrafish. Environ. Sci. Technol 42, 1773–1779. [DOI] [PubMed] [Google Scholar]
- Velki M, Di Paolo C, Nelles J, Seiler TB, Hollert H, 2017. Diuron and diazinon alter the behavior of zebrafish embryos and larvae in the absence of acute toxicity. Chemosphere 180, 65–76. [DOI] [PubMed] [Google Scholar]
- Vignet C, Trenkel VM, Vouillarmet A, Bricca G, Begout ML, Cousin X, 2017. Changes in brain monoamines underlie behavioural disruptions after zebrafish diet exposure to polycyclic aromatic hydrocarbons environmental mixtures. Int. J. Mol. Sci 18, E560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang L, Wang Q, Guo Y, Li N, Ma M, Zhou B, 2016. The neurotoxicity of DE-71: effects on neural development and impairment of serotonergic signaling in zebrafish larvae. J. Appl. Toxicol 36, 1605–1613. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhu Y, Liu C, Liu H, Giesy JP, Hecker M, Lam MH, Yu H, 2012. Accumulation and biotransformation of BDE-47 by zebrafish larvae and teratogenicity and expression of genes along the hypothalamus-pituitary-thyroid axis. Environ. Sci. Technol 46, 12943–12951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


