Abstract
Summary
Background
Anti-tumour necrosis factor-alpha agents (anti-TNF) are effective therapies for the treatment of Crohn’s disease (CD), but their comparative efficacy is unknown.
Aim
To perform a network meta-analysis comparing the efficacy of anti-TNF therapies in CD.
Methods
After screening 506 studies, reviewers extracted information on 10 studies. Traditional meta-analysis (TMA) was used to compare each anti-TNF agent to placebo. Bayesian network meta-analysis (NMA) was performed to compare the effects of anti-TNF agents to placebo. In addition, sample sizes for comparative efficacy trials were calculated.
Results
Compared to placebo, TMA revealed that anti-TNF agents result in a higher likelihood of induction of remission and response (RR: 1.66, 95% CI: 1.17–2.36 and RR: 1.43, 95% CI: 1.17–1.73, respectively) as well as maintenance of remission and response (RR: 1.78, 95% CI: 1.51–2.09 and RR: 1.68, 95% CI: 1.46–1.93, respectively). NMA found nonsignificant trends between infliximab and adalimumab or certolizumab pegol. Among subcutaneous therapies, NMA demonstrated superiority of adalimumab to certolizumab pegol for induction of remission (RR: 2.93, 95% CrI: 1.21–7.75). Sample size calculations suggest that adequately powered head-to-head comparative efficacy trials would require greater than 3000 patients.
Conclusions
All anti-TNF agents are effective for induction and maintenance of response and remission in the treatment of CD. Although adalimumab is superior to certolizumab pegol for induction of remission, there is no evidence of clinical superiority among anti-TNF agents. Head-to-head trials among the anti-TNF agents are impractical in terms of size and cost.
Introduction
Anti-tumour necrosis factor alpha (anti-TNF) therapies are established treatments for moderate to severe Crohn’s disease (CD). Randomised controlled trials of three anti-TNF agents, infliximab (IFX), adalimumab (ADA), and certolizumab pegol (CZP), have demonstrated efficacy over placebo and are FDA approved for the induction and maintenance of clinical response and remission in moderate to severe CD.1–10 However, while these anti-TNF agents are each effective against placebo, whether they share comparable efficacy remains questioned and has not been well studied.
Biological differences among anti-TNF agents allow for potential variability in therapeutic properties and efficacy. IFX is a monoclonal IgG1 antibody with a partially murine anti-TNF Fab region, ADA is an IgG1 antibody containing a humanised Fab region, and CZP is pegylated without an Fc region. Despite these molecular differences, in vitro studies have not demonstrated significant variability in neutralisation of soluble and membrane-bound TNF or modulation of lymphocyte apoptosis between these anti-TNF agents.11, 12 Retrospective and nonrandomised studies have demonstrated IFX and ADA to have similar clinical outcomes in avoidance of corticosteroids, surgery, hospitalisation and improvement in quality of life in patients with CD.13–17 In summary, biologic and retrospective clinical data suggest similar therapeutic activity of these agents in CD.
Head-to-head direct comparative efficacy trials among anti-TNF agents for CD have not been performed. Network meta-analysis (NMA) allows indirect comparisons of individual anti-TNF agents relative to a common comparator (placebo), yielding an estimate of comparative efficacy. We performed both traditional and network meta-analyses of IFX, ADA and CZP clinical trials to assess comparative efficacy for induction and maintenance among anti-TNF agents for CD.
Methods
Data sources and search
The study was conducted in accordance with the PRISMA statement.18 PubMed and Embase databases were the primary sources to identify relevant published, placebo-controlled, randomised clinical trials of anti-TNF agents for CD. A search of human studies in these databases from inception through 31 August 2013 was performed using controlled vocabulary descriptors (Medical Subject Headings and Emtree) and specific keywords to represent the concept of CD and therapeutic use of anti-TNF agents. The studies of interest were placebo-controlled, randomised studies; retrospective and observational studies were not included in any of the analyses.
The search was augmented by manual searches of reference lists from potentially relevant papers to identify additional studies that may have been missed using the computer-assisted strategy. Additionally, all available guidelines, systematic reviews, and meta-analyses pertaining to the therapeutic use of anti-TNF agents in CD were reviewed for any additional potentially relevant studies. The search was not limited by language, though a large majority of the manuscripts were originally published in English.
Study selection
Two investigators (TL, RS) independently reviewed the titles of all identified citations to generate a list of potentially relevant articles for further review. The abstracts of these articles were reviewed to identify studies suitable for inclusion in our final analyses. For a manuscript to be eligible for our study, it had to satisfy the following eligibility criteria: (i) studies had to examine the effect of a single anti-TNF agent on induction and/or maintenance of response or remission in CD; (ii) the treatment of interest was anti-TNF agent monotherapy at standard dosing, although pre-existing concominate therapies were permitted; (iii) studies could not duplicate data already published; (iv) studies were published as full manuscripts; (v) response or remission was defined by a standardised scoring criteria (typically using the Crohn’s Disease Activity Index – CDAI); (vi) studies were placebo-controlled, randomised clinical trials with treatment and control arms. We did not include nonrandomised controlled trials given the concern for study heterogeneity.
Data extraction
Two authors (TL and RS) independently extracted data from the included studies via manual review. Discrepancies between data extracted were resolved via consensus. The following data points were extracted for each study: first author; year of publication; number of centres involved (if multi-centre); drug studied, dosage and dose interval; blinding and randomisation; clinical endpoints (induction or maintenance of either clinical response or remission); presence or absence of concomitant glucocorticoid or immunosuppressive exposure; prior anti-TNF agent exposure status; length of follow-up; presence of a drug washout period; numbers of patients in the treatment and control arms; numbers of patients in each arm who achieved induction of response, induction of remission, maintenance of response, or maintenance of remission; measurement of the primary outcome.
Clinical endpoints
We extracted data to evaluate four clinical endpoints: (i) Induction of Remission – defined as attainment of a CDAI score of less than 150 within 12 weeks of initiation of treatment; (ii) Induction of Response – defined as a decrease in the CDAI score of ≥100 or ≥70 points (based on aim of study) from the baseline score within 12 weeks of initiation of treatment; (iii) Maintenance of Remission – remission (as defined above) maintained at 24–30 weeks. While some studies included 52 week data, more complete data were available for 24–30 week time frames, permitting a more homogenous endpoint comparison between studies; (iv) Maintenance of Response – response (as defined above) maintained for 24–30 weeks.
Quality assessment
Two investigators (AD, DS) critically appraised and quality-rated all eligible studies. The randomised controlled trials were assessed by criteria set forth by the Evidence-Based Gastroenterology Steering Group (EBGSG).19 These criteria were: (i) concealed random allocation; (ii) blinding of patients and caregivers; (iii) equal use of co-interventions for the treatment and placebo groups; (iv) complete follow-up of study patients; and (v) use of an intention-to-treat analysis. Discrepancies in quality assessment were resolved by consensus.
Data synthesis and analysis
The outcomes analysed included induction and maintenance of clinical response or remission in CD. Traditional meta-analysis was used for the direct pairwise comparisons of each anti-TNF vs. placebo and was performed using random-effects meta-analysis techniques in Stata 13.1 (StataCorp, College Station, TX, USA). The differences between random effects and fixed effects were also evaluated when only a single study for a particular drug was evaluated. The Cochran Q-test and I2 inconsistency statistic were used to assess for statistical heterogeneity between trials. When heterogeneity was present, meta-influence analysis and Galbraith plot assessment were performed to identify responsible outlier studies. Pooled relative risks (RRs) and their 95% confidence intervals (95% CIs) were estimated for the various anti-TNF agents.
To compare the efficacy of the anti-TNF agents, a Bayesian network meta-analysis (NMA) was performed with the GeMTC GUI statistical package.20 This form of meta-analysis allows for the analysis of both direct and indirect comparisons and generates estimates of effect (with 95% credible intervals) for all possible pairwise comparisons despite not being evaluated directly in a head-to-head fashion in the included clinical trials. The technique of NMA, in this situation, allows for the formation of indirect comparisons between anti-TNF agents using placebo as a common comparator. In addition, the analysis allows for the ranking of different interventions in order to evaluate the comparative efficacy. For each individual analysis, simulations were repeated 50 000 times to allow convergence and an additional 50 000 simulations were performed to produce the probability statements. Convergence of iterations was evaluated using Gelman-Rubin-Brooke statistic. For this analysis, Markov chain Monte Carlo simulations were utilised to estimate posterior distributions. As direct head-to-head comparative data are lacking, we conservatively chose to use a non-informative uniform prior distribution of effect sizes and precision in this NMA.
Sensitivity analyses and sample size estimates
To assess the robustness of the results, separate traditional meta-analyses were repeated after eliminating statistical heterogeneity by removing outlier studies if any, and excluding studies that did not require response before randomisation into the maintenance study. In instances where nonstandard induction regimens were used in maintenance studies, a sensitivity analysis was performed. Based on the results of this NMA, sample sizes for between-drug comparative effectiveness studies were calculated with the sampsi command in Stata 13.1, assuming 80% power and a 2-sided alpha of 0.05.
Results
Literature search
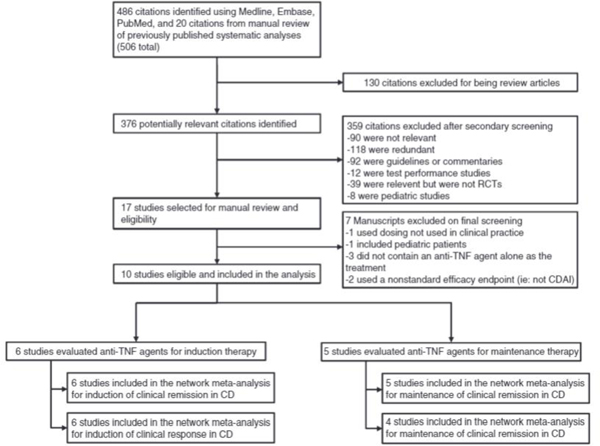
A flow diagram depicting the search and selection process is provided in Figure 1. Initial searches of the Medline and Embase databases yielded 486 citations. Manual search of the PubMed database for pertinent systematic reviews, meta-analyses, and guidelines identified 4 summary documents, a review of which yielded 20 additional citations for a total of 506 citations. Title review of these two groups of citations yielded 376 unique potentially relevant articles. Abstract and/or brief manuscript review of these articles yielded 17 manuscripts appropriate for detailed evaluation. Ten of the remaining manuscripts were included in the final analysis. There was 100% agreement between reviewers regarding final study selection.
Figure 1.
Study inclusion protocol for induction and maintenance of clinical response and remission in Crohn’s disease.
Characteristics of included studies
Characteristics of the included studies are listed in Table 1. The 10 studies meeting eligibility criteria included a total of 1771 subjects for induction and 1690 subjects for maintenance. No comparative effectiveness studies were identified; all included studies compared placebo to various anti-TNF therapies in CD. Two studies compared IFX to placebo. Among these, one study evaluated remission and response as endpoints for induction (n = 52), and the other study evaluated only remission as an endpoint for maintenance (n = 223).1, 2 Four studies compared ADA to placebo, of which two evaluated remission and response as an endpoint for induction (n = 475) and two evaluated remission and response as an endpoint for maintenance (n = 379).4, 6, 7, 9 Four studies compared CZP to placebo, of which three evaluated remission and response as an endpoint for induction (n = 1244) and two evaluated remission and response as an endpoint for maintenance (n = 1088).3, 5, 8, 10 Results of the selected trials are summarised in Table 2.
Table 1 |.
Characteristics of the included studies for the use of anti-TNF agents in the treatment of Crohn’s disease
| Study | Drug | Dosage | Interval | Baseline meds allowed |
Immunosuppressant use (Ctrl%/Tx%) |
Prev anti-TNF: Ctrl/Tx/ Washout |
Quality score |
|---|---|---|---|---|---|---|---|
| Induction of clinical remission in Crohn’s disease | |||||||
| Hanaeur et al. 20064 (CLASSIC-1) | Adalimumab | 160 mg, 80 mg SC | Weeks 0 and 2 | 5-ASA, CCS, AZA, MCP, MTX, ABX | AZA: 18%/14% MCP: 11%/13% MTX: 1%/1% CCS: 34%/32% |
Not allowed | 5 |
| Sandborn et al. 20077 (GAIN) | Adalimumab | 160 mg, 80 mg SC | Weeks 0 and 2 | 5-ASA, CCS, AZA, MCP, MTX, ABX | Any ISf: 51%/46% CCS: 44%/35% |
100%/100%/ 2 month washout | 5 |
| Sandborn et al. 20075 (PRECISE-I) | Certolizumab Pegol | 400 mg SC | Weeks 0, 2 and 4 | 5-ASA, CCS, AZA, MCP, MTX, ABX | Any IS: 20%/21% CCS: 40%/39% |
26%/30%/ 3 month washout | 5 |
| Sandborn et al. 201110 | Certolizumab Pegol | 400 mg SC | Weeks 0, 2 and 4 | 5-ASA, CCS, Immunosup- presants*, ProBX | Any IS: 31%/35% CCS: 46%/44% |
Not allowed | 5 |
| Schreiber et al. 20053 | Certolizumab Pegol | 400 mg SC | Weeks 0, 4 and 8 | 5-ASA, CCS, AZA, MCP, MTX | AZA: 23%/31% MCP: 6%/3% MTX: 7%/4% CCS: 40%/31% |
22%/12%/ 3 month washout | 4 |
| Targan et al. 19971 | Infliximab | 5 mg/kg IV | Week 0 | 5-ASA, CCS, AZA, MCP | AZA: 28%/19% MCP: 16%/15% CCS: 64%/56% |
anti-TNF agents unavailable | 4 |
| Induction of clinical response in Crohn’s disease | |||||||
| Hanaeur et al. 20064 (CLASSIC-1) | Adalimumab | 160 mg, 80 mg SC | Weeks 0 and 2 | 5-ASA, CCS, AZA, MCP, MTX, ABX | AZA: 18%/14% MCP: 11%/13% MTX: 1%/1% CCS: 34%/32% |
Not allowed | 5 |
| Sandborn et al. 20077 (GAIN) | Adalimumab | 160 mg, 80 mg SC | Weeks 0 and 2 | 5-ASA, CCS, AZA, MCP, MTX, ABX | Any ISf: 51%/46% CCS: 44%/35% |
100%/100%/ 2 month washout | 5 |
| Sandborn et al. 20075 (PRECISE-I) | Certolizumab Pegol | 400 mg SC | Weeks 0, 2 and 4 | 5-ASA, CCS, AZA, MCP, MTX, ABX | Any IS: 20%/21% CCS: 40%/39% |
26%/30%/ 3 month washout | 5 |
| Sandborn et al. 201110 | Certolizumab Pegol | 400 mg SC | Weeks 0, 2 and 4 | 5-ASA, CCS, Immunosup- presants*, ProBX | Any IS: 31%/35% CCS: 46%/44% |
Not allowed | 5 |
| Schreiber et al. 20053 | Certolizumab Pegol | 400 mg SC | Weeks 0, 4 and 8 | 5-ASA, CCS, AZA, MCP, MTX | AZA: 23%/31% MCP: 6%/3% MTX: 7%/4% CCS: 40%/31% |
22%/12%/ 3 month washout | 4 |
| Targan et al. 19971 | Infliximab | 5 mg/kg IV | Week 0 | 5-ASA, CCS, AZA, MCP | AZA: 28%/19% MCP: 16%/15% CCS: 64%/56% |
anti-TNF agents unavailable | 4 |
| Maintenance of Clinical Remission in Crohn’s Disease | |||||||
| Colombel et al. 20079 (CHARM) | Adalimumab | 40 mg SC | Every 2 weeks | 5-ASA, CCS, AZA. MCP, MTX, ABX | Any IS: 77.1%/79.1% | 47.6%/49.3%/ 3 month washout | 5 |
| Sandborn et al. 20076 (CLASSIC-II) | Adalimumab | 40 mg SC | Every 2 weeks | 5-ASA, CCS, AZA, MCP, MTX, ABX | AZA: 6%/21% MCP: 6%/0% MTX: 6%/0% CCS: 56%/47% |
Not allowed | 5 |
| Sandborn et al. 20075 (PRECISE-I) | Cetrolizumab Pegol | 40 mg SC | Every 4 weeks | 5-ASA, CCS, AZA, MCP, MTX, ABX | Any IS: 20%/21% CCS: 40%/39% |
26%/30%/ 3 month washout | 5 |
| Schreiber et al. 20078 (PRECISE-II) | Certolizumab Pegol | 400 mg SC | Every 4 weeks | 5-ASA, CCS, AZA, MCP, MTX, ABX | Any IS: 41%/40% CCS: 37%/35% | 24%/24%/ 3 month washout | 5 |
| Hanauer et al. 20022 (ACCENT-I) | Infliximab | 5 mg/kg IV | Every 8 weeks | 5-ASA, CCS, AZA, MCP, MTX, ABX | Immunosuppressant use data not available | Not allowed | 5 |
| Maintenance of clinical response in Crohn’s disease | |||||||
| Colombel et al. 20079 (CHARM) | Adalimumab | 40 mg | Every 2 weeks | 5-ASA, CCS, AZA, MCP, MTX, ABX | Any IS: 77.1%/79.1% | 47.6%/49.3%/ 3 month washout | 5 |
| Sandborn et al. 20076 (CLASSIC-II) | Adalimumab | 40 mg | Every 2 weeks | 5-ASA, CCS, AZA, MCP, MTX, ABX | AZA: 6%/21% MCP: 6%/0% MTX: 6%/0% CCS: 56%/47% |
Not allowed | 5 |
| Sandborn et al. 20075 (PRECISE-I) | Certolizumab Pegol | 400 mg | Every 4 weeks | 5-ASA, CCS, AZA, MCP, MTX, ABX | Any IS: 20%/21% CCS: 40%/39% |
26%/30%/ 3 month washout | 5 |
| Schreiber et al. 20078 (PRECISE-II) | Certolizumab Pegol | 400 mg | Every 4 weeks | 5-ASA, CCS, AZA, MCP, MTX, ABX | Any IS: 41%/40% CCS: 37%/35% |
24%/24%/ 3 month washout | 5 |
CCS, corticosteroids; 5-ASA, 5-aminosalicylates; MCP, mercaptopurine; AZA, azathioprine; MTX, methotrexate; ABX, antibiotics; ProBX, probiotics.
Clinical remission by CDAI (Crohn’s Disease Activity Index) defined as score <150 points. Clinical response by CDAI (Crohn’s disease activity index) defined as a reduction of >100 points, except in Targan et al. 1997, where response was defined by a decrease >70 points. Washout in all cases refers to absence period free of anti-TNF agents if previous receipt of Anti-TNF agents was allowed. Acronyms of studies are provided in parentheses, if available.
In Sandborn et al. 2011, immunosuppressants were allowed concomitant to the study. However, specific drugs were not further specified.
Any IS signifies concurrent use of any of the immunosuppressants: methotrexate, mercaptopurine < gory or aziothioprine (this cate- excludes 5-Aminosalicylates and glucocorticoids).
Table 2 |.
Efficacy data of the included studies for the use of anti-TNF agents in Crohn’s disease
| Study | Drug | Endpoint | Study design | Follow-up (weeks) |
Results |
|
|---|---|---|---|---|---|---|
| Control (%) | Anti-TNF (%) | |||||
| Induction of clinical remission in Crohn’s disease | ||||||
| Hanaeur et al. 2006’4 (CLASSIC-1) | Adalimumab | Remission (CDAI) | All subjects included | 4 | 9/74 (12.2) | 27/76 (35.5) |
| Sandborn et al. 20077 (GAIN) | Adalimumab | Remission (CDAI) | All subjects included | 4 | 12/166 (7.2) | 34/159 (21.4) |
| Sandborn et al. 20075 (PRECISE-I) | Certolizumab Pegol | Remission (CDAI) | All subjects included | 6 | 57/329 (17.3) | 71/331 (21.4) |
| Sandborn et al. 201110 | Certolizumab Pegol | Remission (CDAI) | All subjects included | 6 | 53/215 (24.6) | 68/223 (30.5) |
| Schreiber et al. 20053 | Certolizumab Pegol | Remission (CDAI) | All subjects included | 12 | 17/73 (23.3) | 19/73 (26.0) |
| Targan et al. 19971 | Infliximab | Remission (CDAI) | All subjects included | 12 | 2/25 (8.0) | 8/27 (29.6) |
| Induction of clinical response in Crohn’s disease | ||||||
| Hanaeur et al. 20064 (CLASSIC-1) | Adalimumab | Response (CDAI Decrease by 100) | All subjects included | 4 | 18/74 (24.3) | 38/76 (50.0) |
| Sandborn et al. 20077 (GAIN) | Adalimumab | Response (CDAI Decrease by 100) | All subjects included | 4 | 41/166 (24.7) | 61/159 (38.4) |
| Sandborn et al. 20075 (PRECISE-I) | Certolizumab Pegol | Response (CDAI Decrease by 100) | All subjects included | 6 | 87/329 (26.4) | 115/331 (34.7) |
| Sandborn et al. 201110 | Certolizumab Pegol | Response (CDAI Decrease by 100) | All subjects included | 6 | 71/215 (33.0) | 87/223 (39.0) |
| Schreiber et al. 20053 | Certolizumab Pegol | Combined endpoint: response (CDAI decrease by 100)† remission (CDAI)* | All subjects included | 12 | 26/73 (35.5) | 32/73 (43.8) |
| Targan et al. 19971 | Infliximab | Response (CDAI Decrease by 70) | All subjects included | 12 | 3/25 (12.0) | 13/27 (48.1) |
| Maintenance of clinical remission in Crohn’s disease | ||||||
| Colombel et al. 20079 (CHARM) | Adalimumab | Remission (CDAI) | Responders only | 26 | 29/170 (17.1) | 68/172 (39.5) |
| Sandborn et al. 20076 (CLASSIC-II) | Adalimumab | Remission (CDAI) | Responders only | 24 | 9/18 (50.0) | 16/19 (84.2) |
| Sandborn et al. 20075 (PRECISE-I) | Certolizumab Pegol | Remission (CDAI) | All subjects included | 26 | 32/329 (9.7) | 47/331 (14.2) |
| Schreiber et al. 20078 (PRECISE-II) | Certolizumab Pegol | Remission (CDAI) | Responders only | 26 | 60/212 (28.3) | 103/216 (47.7) |
| Hanauer et al. 20022 (ACCENT-1) | Infliximab | Remission (CDAI) | Responders only | 30 | 23/110 (20.9) | 44/113 (38.9) |
| Maintenance of clinical response in Crohn’s disease | ||||||
| Colombel et al. 20079 (CHARM) | Adalimumab | Response (CDAI) | Responders only | 26 | 45/170 (26.5) | 89/172 (51.7) |
| Sandborn et al. 20076 (CLASSIC-II) | Adalimumab | Response (CDAI) | Responders only | 24 | 11/18 (61.1) | 16/19 (84.2) |
| Sandborn et al. 20075 (PRECISE-I) | Certolizumab Pegol | Response (CDAI) | All subjects included | 26 | 52/329 (15.8) | 75/331 (22.7) |
| Schreiber et al. 20078 (PRECISE-II) | Certolizumab Pegol | Response (CDAI) | Responders only | 26 | 76/212 (35.8) | 135/216 (62.5) |
Clinical remission by CDAI (Crohn's disease activity index) defined as score <150 points. Clinical response by CDAI defined as a reduction of ≥100 points, except in Targan et al. 1997, where response was defined by a decrease ≥70 points. For the study design column, 'All subjects included* means that the outcome of interest was measured among all trial participants. For the study design column, 'Responders only' means that the outcome of interest was measured only among those found to have had a positive response by CDAI score (reduction in CDAI by 70 points) within 4 weeks (people who did not achieve this, nonresponders, were not analysed in the outcome of interest). Acronyms of studies are provided in parentheses if available.
In Schreiber et al. 2005, this result was a combined endpoint of clinical response and clinical remission (response and remission defined as above).
Testing for heterogeneity between eligible studies
Pooled analysis of the effects of IFX, ADA and CZP on induction (remission and response) and maintenance (remission and response) demonstrated no significant statistical heterogeneity among anti-TNF agents.
Meta-analysis results
Induction of remission or response
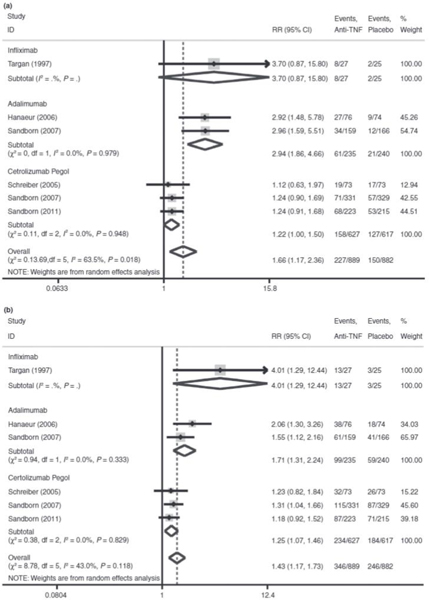
Compared to placebo, traditional meta-analysis revealed that anti-TNF agents result in a 1.66-fold higher likelihood of induction of remission (95% CI: 1.17–2.36) and 1.43-fold higher likelihood of induction of response (95% CI: 1.17–1.73) compared to placebo. IFX resulted in a 3.70-fold higher likelihood of inducing remission (95% CI: 0.87–15.80) and 4-fold higher likelihood of inducing response (95% CI: 1.29–12.44) compared to placebo. ADA resulted in a 2.94-fold higher likelihood of inducing remission (95% CI: 1.86–4.66) and 1.71-fold higher likelihood of inducing response (95% CI: 1.31–2.24) compared to placebo. CZP resulted in a 1.22-fold higher likelihood of inducing remission (95% CI: 1.00–1.50) and 1.25-fold higher likelihood of inducing response (95% CI: 1.07–1.46) compared to placebo. (Figure 2a,b).
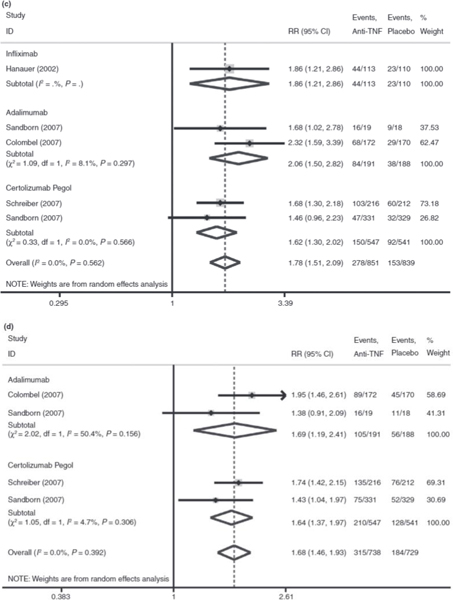
Figure 2.
Panel: Meta-analysis of anti-TNFs for the treatment of Crohn’s disease. (a) Meta-analysis of the induction of remission endpoint. (b) Meta-analysis of the induction of response endpoint. (c) Meta-analysis of the maintenance of remission endpoint. (d) Meta-analysis of the maintenance of response endpoint.
Network meta-analysis of agents for induction of remission demonstrated trends of IFX being superior to ADA (RR: 1.52 for IFX vs. ADA, 95% CrI: 0.20–17.46) and CZP (RR: 4.29 for IFX vs. CZP, 95% CrI: 0.65–46.09), but these results did not reach statistical significance. Between subcutaneous anti-TNF agents, it is notable that ADA was superior to CZP in the induction of remission (RR: 2.93 for ADA vs. CZP, 95% CrI: 1.21–7.75). Rank order analysis demonstrated that IFX was the most effective in 66.7% of simulations, ADA was most effective in 33.3% of simulations and CZP was not most effective in any simulations.
The network meta-analysis of agents for the induction of response suggested IFX was superior to both ADA and CZP, however these trends did not reach statistical significance (RR: 3.17 for IFX vs. ADA, 95% CrI: 0.53–22.96; RR: 5.36 for IFX vs. CZP, 95% CrI: 0.91–40.15). Among subcutaneous anti-TNF treatments for induction of response, neither was shown to be significantly superior (RR: 1.73 for ADA vs. CZP, 95% CrI: 0.69–4.25). IFX was ranked the most effective drug in 87% of the simulations, while ADA was favoured in 12% and CZP in 1%.
Maintenance of remission or response
Compared to placebo, traditional meta-analysis revealed that anti-TNF agents result in a 1.78-fold higher likelihood of maintenance of remission (95% CI: 1.51–2.09) and 1.68-fold higher likelihood of maintenance of response (95% CI: 1.46–1.93) compared to placebo. IFX resulted in a 1.86-fold higher likelihood of maintaining remission compared to placebo (95% CI: 1.21–2.86). ADA resulted in a 2.06-fold higher likelihood of maintaining remission (95% CI: 1.50–2.82) and 1.69-fold higher likelihood of maintaining response (95% CI: 1.19–2.41) compared to placebo. CZP resulted in a 1.62-fold higher likelihood of maintaining remission (95% CI: 1.30–2.02) and 1.64-fold higher likelihood of maintaining response (95% CI: 1.37–1.97) compared to placebo (Figure 2c,d).
Network meta-analysis of agents for maintenance of remission did not show significant difference between agents (ADA vs. IFX RR: 1.42, 95% CrI: 0.17–9.27; IFX vs. CZP RR: 1.23, 95% CrI: 0.26–13.14; ADA vs. CZP RR: 1.81, 95% CrI: 0.55–8.51). ADA was ranked the most effective drug in 63% of simulations, IFX in 29% and CZP in 7%.
Finally, the network meta-analysis of agents for the maintenance of response demonstrated no statistically significant difference between agents ADA vs. CZP (RR: 1.45, 95% CrI: 0.36–6.08); compatible IFX data were not available. ADA was ranked the most effective drug in 75% of simulations and CZP ranked first in 25% of simulations.
Direct comparison sample size estimations
Using data generated by our NMA as a measure of effect size, the required sample sizes for direct comparative effectiveness trials between anti-TNF agents are large, requiring over 3000 subjects (Table 3).
Table 3.
Total number of subjects required for comparative efficacy RCTs between anti-TNF agents for CD induction and maintenance of remission
| Total subject number (induction/maintenance) | Infliximab | Certolizumab pegol | Adalimumab |
|---|---|---|---|
| Infliximab | – | 3272/558 | 4780/3076 |
| Certolizumab pegol | 3272/558 | – | 104518/286 |
| Adalimumab | 4780/3076 | 104518/286 | – |
Publication bias
The funnel plot asymmetry test for publication bias using the Harbord test was negative for induction of remission (P = 0.12, n = 6), maintenance of remission (P = 0.96, n = 5), and maintenance of response (P = 0.34, n = 4). However, asymmetry testing approached significance for induction of response (P = 0.053, n = 6), likely due to the small enrolment in the Targan et al. study resulting in an inflated treatment effect.1
Sensitivity analysis
The sensitivity analyses using traditional meta-analyses did not substantively change the results. Specifically, excluding studies that did not require response before randomisation into the maintenance study (n = 1, Sandborn et al. 20075), and maintenance studies that used nonstandard induction regimens (n = 1, Colombel et al. 20079).
Discussion
Comparative efficacy of anti-TNF therapies for Crohn’s disease remains a commonly debated topic with great implications for treatment algorithms when considering which anti-TNF to utilise first for an individual patient. Further, the near-term entry of several new biological and small molecule therapies for CD will raise questions of how to optimally sequence the variety of new therapeutic mechanisms in CD. Traditional analysis of comparable anti-TNF clinical trials presented here reinforces the efficacy of this medication class, echoing the results of prior meta-analyses.21, 22 To our knowledge, this study is the first to assess comparative efficacy of anti-TNF agents in CD through a network meta-analysis. Using non-informative priors for conservative results, no individual anti-TNF agent was shown to be statistically superior for remission or maintenance of CD by CDAI. Rank order analysis showed higher remission and response rates for induction using IFX, while ADA was favoured for maintenance of remission therapy, although the 95% credibility interval crossed 1. These findings suggest potential variable efficacy, yet sample sizes required to detect such differences as part of a head-to-head trial are impractically large.
Several open-label studies have evaluated comparative efficacy of anti-TNF therapies in Crohn’s disease. Single centre nonrandomised open-label cohort studies have shown comparable efficacy and safety between IFX and ADA at 1 year.23 Yet, issues of small study size, the absence of randomisation, and the lack of objective assessments of disease activity preclude firm conclusions on comparative efficacy from these data. Further, one would expect that if anti-TNF agents have no significant difference in efficacy that they should be interchangeable. In-class anti-TNF switching following secondary loss of response due to anti-drug antibody formation has been shown to re-establish disease control, but this point does not necessarily support true inter-changeability of anti-TNF therapies.24 Van Assche and colleagues investigated the impact of elective switching of anti-TNF agents in a randomised open-label trial substituting IFX with ADA in subjects with clinically controlled CD.25 A significant portion of patients returned to IFX after 1 year due to disease-related complications while on ADA, despite allowing for dose escalation and optimisation in both groups and observing stable anti-TNF drug levels. While the SWITCH study was not designed with the intent of determining comparative efficacy between IFX and ADA, these data raise doubts that anti-TNF therapies are completely equivalent in CD.
Absent clear evidence of an individual anti-TNF demonstrating superior efficacy in CD, safety, cost and patient preference considerations impact the initial choice of anti-TNF. Safety is believed comparable between anti-TNFs, comprehensively evaluated in recent meta-analyses of various adverse effects such as the risk of melanoma,26 opportunistic infections27 and lymphoma.28 Some data suggest IFX may have higher rates of attenuation of response and intolerance.29 While prospective registry studies of maternal–foetal outcomes following exposure to anti-TNF continue to collect data, CZP has been shown to have lower placental transfer, which subsequently could confer a safety advantage for women planning pregnancies; a considerable consideration given the demographic of young, fertile women with CD.30
Anti-TNF therapies have been shown to reduce the overall economic burden of Crohn’s disease, offsetting their high cost over time.31, 32 However, infusion-related costs are frequently cited as economic reasons to consider subcutaneous therapy. Retrospective studies in the United States and United Kingdom have suggested that switching from IFX to ADA could result in an annual cost savings of US$7000 without increasing disease-related expenditures in hospitalisation, surgery, or diagnostics, even when allowing for nonsystematic ADA dose escalation.33, 34 Subcutaneous anti-TNF may have an economic advantage over infused agents, though more detailed studies of the financial impact of dose escalation are needed. Finally, patient surveys in both IBD and rheumatoid arthritis repeatedly report that after side effect profile, route of administration is the next most important factor with patients preferring subcutaneous over infusion based anti-TNF therapies.35, 36
The limitations of current clinical trial endpoints, as well as those of NMA, must be considered when interpreting the presented results. The utility of CDAI as a measure of disease activity has been increasingly questioned. Shortcomings of CDAI include its lack of concordance with objective measures of disease activity, poor prediction of prolonged remission, and limited reproducibility.37, 38 Further, phenotypic heterogeneity of CD, including inflammatory and fibrostenotic features, is not well characterised by CDAI. These limitations prevent accurate assessment of the inflammatory burden of disease activity, which is most amenable to the therapies being evaluated. Objective endpoints of mucosal inflammation represent a more reproducible and prognostic measure of therapeutic efficacy in IBD. Endoscopic scoring in conjunction with complementary biomarkers and incorporation of patient-reported outcomes (PRO) instruments are increasing being utilised to comprehensively assess therapeutic efficacy and represent the future of disease activity assessment.39, 40 C-reactive protein (CRP) measurements were reported in a portion of the included trials, however neither data collection nor stratification by CRP was uniform across the included studies. Regardless of methodological rigour, CDAI is neither sufficiently accurate nor reproducible as a measure of disease activity and is a significant limitation to any comparative analysis in CD.
Despite refining the list of included studies in this NMA to randomised placebo-controlled trials with conventional anti-TNF dosing using a common shared endpoint, variations in study protocol may have impacted the results. Studies did not stratify by disease phenotype or fistula activity; thought one study (Sandborn et al. 201110) excluded patients with active perianal disease. Studies were not uniform in the explicit exclusion of patients with known or suspected obstructive fibrostenotic strictures. Further, induction regimens were not uniform and varied from current standards, limiting applicability of the results. CHARM (ADA) used an 80 mg/40 mg induction regimen.9 ACCENT-I (IFX) completed standard induction of 5 mg/kg at 0, 2 and 6 weeks, but the decision to continue into the maintenance phase was made based on clinical response at week 2 after a single 5 mg/kg infusion.2 CLASSIC-II (ADA) required clinical remission for randomisation into the maintenance study, potentially selecting for subjects with a more robust clinical response to anti-TNF.6 However, a sensitivity analysis excluding CLASSIC-II data did not meaningfully change the results. Prior anti-TNF exposure also varied between trials. Subjects in all selected IFX studies and CLASSIC-I/II (ADA) studies were anti-TNF naïve. Of those in the PRECISE-I/II CZP studies, approximately 28% of subjects were previously exposed to an anti-TNF, while in the CHARM (ADA) maintenance study 49.6% had prior anti-TNF exposure. Finally, one ADA study required prior IFX use as inclusion criteria.7 While there is no clear bias presented by the selected studies, standardisation or stratification of prior anti-TNF exposure status may have made the indirect comparison more accurate.
Finally, there are several technical aspects that must be considered when using NMA. Without direct comparative data to inform the network, we chose to use non-informative priors for treatment effects and assumed homogeneous variance between studies. This unbiased approach is commonly used in NMA and is considered the most conservative for indirect comparison, but it is subject to increased type-II error. Thorlund and colleagues have used informative priors to estimate variance and improve precision of the analysis.41 Our group recently published a comparative effectiveness study for anti-TNF agents in ulcerative colitis showing a trend of IFX superiority over ADA for induction (RR = 0.46, 95% CrI: 0.10–3.05).42 Thorlund and colleagues also performed NMA yielding a comparable point estimate of IFX superiority over ADA, but using informative priors they reported statistical significance (OR = 0.42, 95% CrI: 0.17–0.97).43 Deciding on whether to use informative priors remains controversial. A recent report by the Agency for Healthcare Research and Quality (AHRQ) evaluated various studies that used mixed treatment comparisons and concluded there is enough data to support using informative priors.44 Choosing non-informative priors for this NMA favours a lower likelihood of type I error (false positive) at the cost of increased type-II error (false negative); this represents the primary bias in our approach.
In conclusion, IFX, ADA and CZP are all effective treatments for induction and maintenance of remission and response in Crohn’s disease. Network meta-analysis did not demonstrate statistically significant therapeutic differences among anti-TNF therapies. In the absence of compelling data demonstrating variable efficacy, factors including safety, cost and patient preference should guide anti-TNF choice and sequencing. The large sample sizes required to demonstrate differences among anti-TNFs make these trial comparisons impractical and unlikely to ever occur, lending value to our NMA, though NMA may be insensitive to small differences in clinical efficacy in the absence of direct comparative trial data to inform the network. Future therapeutic trials in CD using objective quantitative measures of disease activity such as endoscopic and radiographic scoring, may allow indirect simulation-based comparisons like NMA to better approximate true comparative efficacy.45, 46
Acknowledgements
Declaration of funding interests: Crohn’s and Colitis Foundation of America (Stidham), Veterans Affairs HSR&D Career Development Awards (Waljee, Saini), American Cancer Society, Bankhead-Coley Team Science Program, Sylvester Comprehensive Cancer Center, Olympus America (Sussman). The content is solely the responsibility of the authors and does not necessarily represent the official views of the VA.
Footnotes
Declaration of personal interests: None.
References
- 1.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997; 337: 1029–35. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359: 1541–9. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology 2005; 129: 807–18. [DOI] [PubMed] [Google Scholar]
- 4.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130: 323–33. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med 2007; 357: 228–38. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007; 56: 1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 2007; 146: 829–38. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med 2007; 357: 239–50. [DOI] [PubMed] [Google Scholar]
- 9.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007; 132: 52–65. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Schreiber S, Feagan BG, et al. Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol 2011; 9: 670–8.e3. [DOI] [PubMed] [Google Scholar]
- 11.Kaymakcalan Z, Sakorafas P, Bose S, et al. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol 2009; 131: 308–16. [DOI] [PubMed] [Google Scholar]
- 12.Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis 2007; 13: 1323–32. [DOI] [PubMed] [Google Scholar]
- 13.Chen JS, Makovey J, Lassere M, Buchbinder R, March LM. Comparative effectiveness of anti-tumour necrosis factor (TNF) drugs on health-related quality of life among patients with inflammatory arthritis. Arthritis Care Res (Hoboken) 2014; 66: 464–72. [DOI] [PubMed] [Google Scholar]
- 14.Kestens C, van Oijen MG, Mulder CL, et al. Adalimumab and infliximab are equally effective for Crohn’s disease in patients not previously treated with anti-tumor necrosis factor-alpha agents. Clin Gastroenterol Hepatol 2013; 11: 826–31. [DOI] [PubMed] [Google Scholar]
- 15.Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn’s Disease. Clin Gastroenterol Hepatol 2014; 12: 811–7.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patil SA, Rustgi A, Langenberg P, Cross RK. Comparative effectiveness of anti-TNF agents for Crohn’s disease in a tertiary referral IBD practice. Dig Dis Sci 2013; 58: 209–15. [DOI] [PubMed] [Google Scholar]
- 17.Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol 2009; 2: 101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenfeld P, Cook D, Hamilton F, Laine L, Morgan D, Peterson W. An evidence-based approach to gastroenterology therapy. Evidence-Based Gastroenterology Steering; [DOI] [PubMed] [Google Scholar]
- 20.van Valkenhoef G, Guobing L, de Brock B, et al. Automating network meta-analysis. Research Synthesis Methods 2012; 3: 285–99. [DOI] [PubMed] [Google Scholar]
- 21.Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 644–59. [DOI] [PubMed] [Google Scholar]
- 22.Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol 2008; 6: 644–53. [DOI] [PubMed] [Google Scholar]
- 23.Zorzi F, Zuzzi S, Onali S, et al. Efficacy and safety of infliximab and adalimumab in Crohn’s disease: a single centre study. Aliment Pharmacol Ther 2012; 35: 1397–407. [DOI] [PubMed] [Google Scholar]
- 24.Peyrin-Biroulet L, Laclotte C, Bigard MA. Adalimumab maintenance therapy for Crohn’s disease with intolerance or lost response to infliximab: an open-label study. Aliment Pharmacol Ther 2007; 25: 675–80. [DOI] [PubMed] [Google Scholar]
- 25.Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut 2012; 61: 229–34. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Nagpal SJ, Murad MH, et al. Inflammatory bowel disease is associated with an increased risk of melanoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014; 12: 210–8. [DOI] [PubMed] [Google Scholar]
- 27.Targownik LE, Bernstein CN. Infectious and malignant complications of TNF inhibitor therapy in IBD. Am J Gastroenterol 2013; 108: 1835–42. [DOI] [PubMed] [Google Scholar]
- 28.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol 2009; 7: 874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Pan SM, Dehler S, Ciurea A, et al. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 2009; 61: 560–8. [DOI] [PubMed] [Google Scholar]
- 30.Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013; 11: 286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsay JO, Chipperfield R, Giles A, Wheeler C, Orchard T, investigators Is. A UK retrospective observational study of clinical outcomes and healthcare resource utilisation of infliximab treatment in Crohn’s disease. Aliment Pharmacol Ther 2013; 38: 52–61. [DOI] [PubMed] [Google Scholar]
- 32.Loftus EV Jr, Johnson SJ, Yu AP, Wu EQ, Chao J, Mulani PM. Cost-effectiveness of adalimumab for the maintenance of remission in patients with Crohn’s disease. Eur J Gastroenterol Hepatol 2009; 21: 1302–9. [DOI] [PubMed] [Google Scholar]
- 33.Choi GK, Collins SD, Greer DP, et al. Costs of adalimumab versus infliximab as first-line biological therapy for luminal Crohn’s disease. J Crohns Colitis 2014; 8: 375–83. [DOI] [PubMed] [Google Scholar]
- 34.Sussman DA, Kubiliun N, Mulani PM, et al. Comparison of medical costs among patients using adalimumab and infliximab: a retrospective study (COMPAIRS). Inflamm Bowel Dis 2012; 18: 2043–55. [DOI] [PubMed] [Google Scholar]
- 35.Chilton F, Collett RA. Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskeletal Care 2008; 6: 1–14. [DOI] [PubMed] [Google Scholar]
- 36.Scarpato S, Antivalle M, Favalli EG, et al. Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study). Rheumatology (Oxford) 2010; 49: 289–94. [DOI] [PubMed] [Google Scholar]
- 37.Sands BE, Ooi CJ. A survey of methodological variation in the Crohn’s disease activity index. Inflamm Bowel Dis 2005; 11: 133–8. [DOI] [PubMed] [Google Scholar]
- 38.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010; 105: 162–9. [DOI] [PubMed] [Google Scholar]
- 39.Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol 2013; doi: 10.1016/j.cgh.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011; 140: 1817– 26.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorlund K, Druyts E, Avina-Zubieta JA, Wu P, Mills EJ. Why the findings of published multiple treatment comparison meta-analyses of biologic treatments for rheumatoid arthritis are different: an overview of recurrent methodological shortcomings. Ann Rheum Dis 2013; 72: 1524–35. [DOI] [PubMed] [Google Scholar]
- 42.Stidham RW, Lee TC, Higgins PD, et al. Systematic review with network meta-analysis: the efficacy of anti-tumour necrosis factor-alpha agents for the treatment of ulcerative colitis. Aliment Pharmacol Ther 2014; 39: 660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorlund K, Druyts E, Mills EJ, Fedorak RN, Marshall JK. Adalimumab versus infliximab for the treatment of moderate to severe ulcerative colitis in adult patients naive to anti-TNF therapy: an indirect treatment comparison meta-analysis. J Crohns Colitis 2014; doi: 10.1016/j.crohns.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Coleman CI, Phung OJ, Cappelleri JC, et al. Use of Mixed Treatment Comparisons in Systematic Reviews. Rockville, MD: AHRQ Methods for Effective Health Care, 2012. [PubMed] [Google Scholar]
- 45.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm Bowel Dis 2011; 17: 1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rimola J, Rodriguez S, Garcia-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 2009; 58: 1113–20. [DOI] [PubMed] [Google Scholar]