Abstract
Stressful experiences can cause long-lasting sensitization of fear and anxiety that extends beyond the circumstances of the initial trauma. The neural mechanisms of these stress effects have been studied extensively in rats using the stress-enhanced fear learning (SEFL) paradigm, in which exposure to footshock stress potentiates subsequent fear conditioning. Here we establish a mouse version of the SEFL. Male and female 129s6 mice received four 1-mA foot shocks or equivalent context exposure without shock. Shock exposure induced Pavlovian fear conditioning to the shock context and produced three more general effects: (1) suppression of open field exploration, (2) potentiated unconditioned fear of a novel tone stimulus, and (3) enhanced fear conditioning in a novel context. To determine whether these effects of footshock stress reflect generalized Pavlovian fear conditioning versus nonassociative fear sensitization, some mice received extinction training in the footshock stress context, which reduced contextual fear to the levels of unstressed control mice. Extinction restored normal open field exploration, suggesting that this effect of stress reflects generalized Pavlovian fear. In contrast, extinction failed to attenuate stress-enhanced fear, indicating that stress-enhanced fear is nonassociative and mechanistically distinct from Pavlovian fear conditioning. The effects of footshock stress were similar in male and female mice, although female mice displayed larger acute responses to fear-inducing stimuli than did males. The results demonstrate that footshock stress influences emotional behavior through distinct associative and nonassociative mechanisms, which likely involve unique neural underpinnings.
Introduction
Stressful or traumatic experiences can cause long-lasting changes in thought and behavior through two distinct learning mechanisms (Rau, DeCola, & Fanselow, 2005; Seo, Carillo, Chih-Hsiung Lim, Tanaka, & Drew, 2015; Siegmund & Wotjak, 2007a). One is Pavlovian conditioning, which occurs when a previously neutral stimulus becomes associated with a stressor and thereby acquires the ability to evoke fear. The second process is sensitization, which is a generalized, nonassociative change in emotional responsiveness (Mackintosh, 1974). In trauma-and stressor-related disorders such as post-traumatic stress disorder (PTSD), both associative and nonassociative learning mechanisms appear to be recruited (Friedman, Resick, Bryant, & Brewin, 2010). PTSD includes symptoms resembling associative learning, such as exaggerated reactions to cues resembling those present during the original trauma, as well as more generalized symptoms resembling sensitization. These symptoms are classified as hyperarousal symptoms and include hypervigilance, exaggerated startle responses, and irritability.
Stress-enhanced fear learning (SEFL) has become a popular rodent model of PTSD because it captures both nonassociative and associative effects of stress using a simple and well-defined set of behavioral procedures (Perusini et al., 2015; Poulos, Zhuravka, Long, Gannam, & Fanselow, 2015; Rau et al., 2005). In SEFL, a rodent is given several strong footshocks in a single session within a conditioning chamber. The footshock stress causes a variety of long-lasting behavioral consequences, including induction of Pavlovian contextual fear conditioning to the stress context, reduced exploratory behavior in the open field, potentiation of fear to other acute stressors, enhanced Pavlovian fear conditioning to new contexts and cues (Perusini et al., 2015), and increased drug-seeking (Pizzimenti, Navis, & Lattal, 2017). Importantly, stress-induced enhancement of fear conditioning persists even if fear of the original shock context is extinguished (Rau et al., 2005; Rau & Fanselow, 2009). This extinction-resistance suggests that enhanced fear learning does not stem from generalization of the original Pavlovian conditioned fear—it instead represents sensitization, a mechanistically distinct form of plasticity. The extinction resistance is also important from a translational perspective because PTSD itself is at least partially resistant to exposure therapy, a clinical analog of extinction (Bradley, Greene, Russ, Dutra, & Westen, 2005).
The experiments reported here had two purposes. First, we sought to establish a mouse model of SEFL using footshock as the stressor. SEFL was established, and has mainly been applied, in rats. One notable exception is a model developed by Wotjak and colleagues (Siegmund & Wotjak, 2007a). In their model, a single footshock was shown to cause long-lasting suppression of open field exploration (Kamprath & Wotjak, 2004), increased startle (Golub, Mauch, Dahlhoff, & Wotjak, 2009), and enhanced unconditioned fear in mice (Kamprath & Wotjak, 2004). However, the group did not evaluate whether the footshock stress enhances subsequent fear learning. Other mouse models of PTSD rely on more complex or chronic forms of stress, such as restraint (Sillivan et al., 2019; 2017), repeated social defeat (Krishnan et al., 2007) or chronic unpredictable stress (Mineur, Belzung, & Crusio, 2006; Nollet, Le Guisquet, & Belzung, 2013). Although these models have been very useful for characterizing biological effects of stress exposure, the complexity of the stressors in these paradigms make the learning mechanisms involved difficult to decipher. For this reason, the comparative simplicity of the SEFL model is a distinct advantage.
The second purpose to the current studies is to determine whether the behavioral effects of footshock stress in mice are extinction-resistant. In rats, stress-induced enhancement of fear conditioning is insensitive to extinction of the original learned fear (Rau et al., 2005; Rau & Fanselow, 2009), but it is unknown whether extinction training alleviates other effects of stress. In mice, extinction of the original fear can alleviate the stress-induced enhancement of acoustic startle (Golub et al., 2009), but the effects of extinction on other stress phenotypes have not been analyzed. Here, we examine the effects of footshock stress on open field exploration, unconditioned fear, and fear conditioning in 129s6 mice. We demonstrate that extinction alleviates the effects of footshock stress on exploratory behavior but fails to alleviate effects on unconditioned fear and fear conditioning. Our findings demonstrate that stress-enhanced fear in mice reflects sensitization, which is mechanistically distinct from associative fear conditioning.
Methods
Subjects
Male (n = 59) and female (n = 43) mice from the 129S6/SvEvTac background (Taconic, Germantown, NY) were bred in-house and housed groups of 2–4 in plastic cages with wood-chip bedding. Seventy mice (25 female, 45 male) were used in Experiment 1, and 32 mice (18 female, 14 male) were used in Experiment 2. All mice were maintained on a 12 hr light/dark cycle (7:00 A.M. to 7:00 P.M.). Experiments were conducted during the light phase. All procedures were approved by the University of Texas at Austin Institutional Animal Care and Use Committee.
Apparatus
Conditioning took place in a 30.5 × 24 × 21 cm chamber (Med Associates) with three aluminum walls, a clear Plexiglas door, and ceiling and stainless-steel grid flooring (Med Associates VFC-005A; Context A). Each conditioning chamber was placed within a sound-attenuating cabinet equipped with overhead white and infrared lights that were kept on throughout all sessions. Scents were applied to a paper towel in the waste tray below the chamber floor. The chambers could be configured into three distinct contexts (Huckleberry, Ferguson, & Drew, 2016). Context A had a steel bar floor in parallel configuration (Med-Associates VFC-005A), was cleaned before and after each animal with 70% ethanol, and was scented with 1% acetic acid solution. Context B consisted of the same conditioning chamber with wood chip bedding covering a plastic insert placed over the grid flooring. Context B was cleaned and scented with Clorox wipes. Context C consisted of the same chamber with a staggered steel bar floor (Med Associates VFC-005A-S) and an A-Frame insert (Med Associates ENV-008-IRT). Context C was cleaned and scented with Windex cleaning solution.
Freezing behavior was scored continuously throughout all sessions using Video Freeze software (Med-Associates Inc) via digital video cameras mounted on the door of the sound-attenuating cabinets.
Procedures
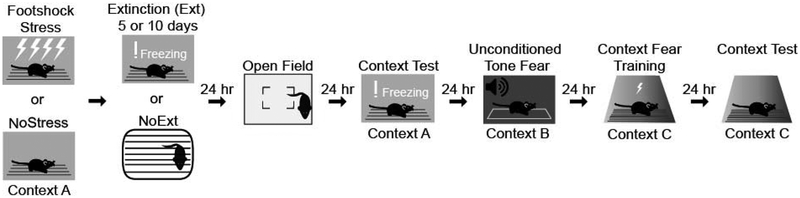
The sequence of behavioral procedures is shown in Figure 1. In brief, mice received footshock stress in Context A. A subset of mice then received 5 (Experiment 1) or 10 (Experiment 2) extinction sessions in Context A. Following extinction, all mice received the following sequence of tests: open field, a test of context-induced fear in Context A, a test of unconditioned tone fear in Context B, single-shock context conditioning in Context C, and a test of context-induced fear in Context C. Mice were moved to a holding room adjacent the testing rooms at least 30 minutes prior to the start of all test sessions. The holding room was lighted dimly, and a white noise generator was operated in the room to mask any sounds associated with testing in the adjacent rooms.
Figure 1.
Schematic of the experimental design and sequence of behavioral assays.
Footshock Stress
Mice received four 2-s, 1 mA foot shocks delivered through the grid flooring in Context A. Shocks were delivered at 160 s, 240 s, 320 s, and 400 s after the mouse was placed into the chamber. Mice were removed 40 s after the final shock.
Extinction Training
Mice were returned to Context A for 5 min with no shock once per day for 5 (Experiment 1) or 10 (Experiment 2) sessions. Following completion of extinction training and open field testing (described below), all mice received a 3-min test of context-elicited fear in Context A.
Open Field
The open field test was performed in four arenas (40 × 40 cm) with opaque gray walls. Arenas were illuminated from above with white incandescent bulbs producing 85 lux at the center of the arena. Mice were placed into the open field apparatus for 30 min. Open field testing occurred in different testing room than that used for fear conditioning. Sessions were recorded by digital video cameras mounted above the arenas and analyzed using AnyMaze software (Stoelting Inc.).
Unconditioned Tone Fear
Mice were moved from the holding room into the testing room in opaque plastic containers. Mice were placed into Context B and a tone (30s, 90 dB, 9 kHz) was played three times with 30-s interstimulus intervals. Mice were removed 30 s after the final tone. Freezing behavior was scored throughout the session. Because freezing during the tone and post-tone periods was similar (Figure S1), for the purpose of analysis we divided the session into two periods: the baseline (Pre-Tone) period, which is the 1 min before the first tone presentation, and the tone period, which includes all time during and after the first tone presentation.
Singe-Shock Contextual Fear Conditioning
Mice were moved from the holding room into the testing room in opaque plastic containers. Mice were placed in the Context C. A single footshock (2 s, 0.5 mA) was delivered through the grid flooring 3 min after session start. Mice were removed from the conditioning chamber 30 s after the shock and returned to the home cage. On the following day (24 h after conditioning), mice were returned into Context C for 3 minutes without shock to measure context-elicited fear.
Statistics
Data were analyzed using ANOVA or repeated-measures (RM) ANOVA as indicated below. Significant main effects and interactions were probed using pairwise Tukey HSD tests for between-subjects comparisons and Bonferroni-adjusted paired t-tests for within-subjects comparisons. Alpha was set at p = .05 for all tests. Statistical analyses were performed in Prism 6 (Graphpad Software) or JMP Pro 14 (SAS Institute Inc.). Full statistical results are reported in Supplementary Tables 1–3.
Results
Experiment 1: 5 Sessions of Extinction Training
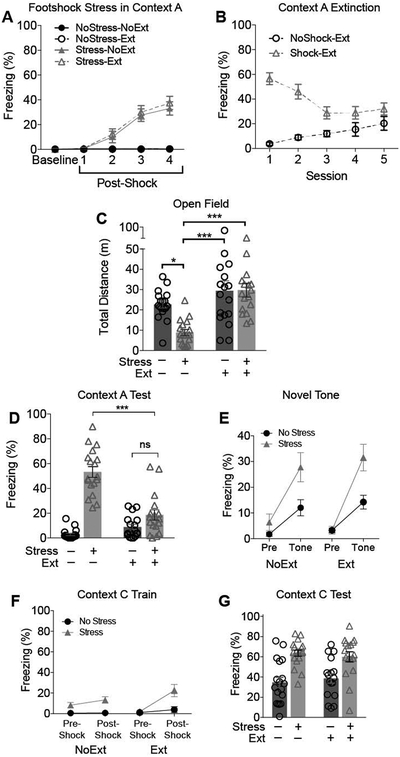
The behavioral procedure is diagrammed in Figure 1A. There were 4 experimental groups: NoStress-NoExt (n= 6 female, 12 male), NoStress-Ext (n= 7 female, 10 male), Stress-NoExt (n= 6 female, 12 male), and Stress-Ext (n= 6 female,11 male). The footshock stress session occurred in Context A on day 1 of the procedure. Mice received either 4 unsignaled footshocks within a single session (Stress Groups) or context exposure with no shocks (NoStress Groups). Freezing behavior increased during the session in Stress groups but remained low in the NoStress groups (Figure 2A). To extinguish contextual fear caused by the footshock stress, mice in Groups Stress-Ext received 5 sessions (one per day) of context exposure without shock (Figure 2B). To control for nonspecific effects of context exposure, Group NoStress-Ext received equivalent context exposure. Groups Stress-NoExt and NoStress-NoExt remained in the vivarium undisturbed during this period. Extinction data were analyzed using Stress X Session RM-ANOVA, which yielded a significant Stress X Session interaction (F4,120 = 17.840, p < .0001). Freezing declined over sessions in Group Stress-Ext (Session 1 v 5: t120 =, 5.969, p < .0001, Bonferroni-corrected). In Group NoStress-Ext, freezing increased modestly over sessions (Session 1 v 5: t120 = 3.794, p = .0009), presumably owing to increased immobility (habituation of exploration) with extended context exposure.
Figure 2.
Results of Experiment 1 (5 sessions of extinction). (A) During the footshock stress session, freezing behavior increased as a function of the 4 shock presentations. (B) During extinction training, freezing declined in Group Stress-Ext but modestly increased in Group NoStress-Ext. (C) Prior footshock stress suppressed open field exploration. This effect was completely reversed by extinction training. (D) Freezing behavior during the test session in the stress context (Context A). Extinction training reduced freezing behavior in stressed mice to the levels of mice that did not receive footshock stress. (E) Prior footshock stress potentiated unconditioned fear of a novel tone. This effect of stress was not attenuated by extinction training. (F) Prior footshock stress increased freezing during contextual fear conditioning in a novel context (Context C). There was no significant effect of extinction training. (G) In the Context C test session, context-elicited fear was enhanced in mice that received prior footshock stress. This effect was not reduced by extinction training. NoStress-NoExt (n=18); NoStress-Ext (n=17); Stress-NoExt (n=18); Stress-Ext (n=17). *p<0.05, **p<0.01, ***p<0.001
Next we sought to (1) confirm that exposure to footshock stress potentiates fear and anxiety-like behavior in other contexts, as previously reported, and (2) determine whether extinction of conditioned fear produced by footshock stress abolishes this potentiation. Consistent with previous reports (Kamprath & Wotjak, 2004; Perusini et al., 2015), footshock stress significantly reduced total locomotion in the open field (Figure 2C). This effect was completely abolished by context extinction training (Shock X Extinction Interaction: F1,1 = 5.172, p = .0262).
Following the open field, all mice received a contextual fear test session in Context A (Figure 2D), to confirm that extinction reduced conditioned fear of Context A. The test confirmed strong contextual fear in Group Stress-NoExt and comparatively reduced freezing in Group Stress-Ext and in the NoStress groups (Shock X Extinction Interaction: F1,1 = 40.218, p < .0001; see also Suppl. Table 1). Freezing levels of Groups Stress-Ext and NoStress-Ext did not differ (Tukey HSD test: p = .1612), indicating that extinction training reduced conditioned fear to the level of unshocked controls.
To evaluate sensitization of unconditioned fear, mice were exposed to a novel tone in a novel context (Context B). We compared freezing behavior before (Pre-Tone) and after the start of the first tone presentation (Tone). Freezing was low in all groups during the pre-tone period and increased after the first tone presentation (Figure 2E). Tone-elicited freezing was greatly enhanced in mice that received prior footshock stress regardless of extinction training. RM-ANOVA confirmed a significant Time Period X Stress interaction (F1,66 = 13.720, p = .0004), but all effects of extinction training were nonsignificant (all F s < 1, see Suppl. Table 1).
The final behavioral assay was a test of stress-enhanced fear learning. Mice received a single mild footshock in a novel context (Context C) followed by a test for context-elicited fear 24h later in Context C. During the training session (Figure 2F), freezing levels were low during the pre-shock period and higher after the shock. The shock-induced increase in freezing was greater in the Stress groups than in the NoStress groups (Time Period X Stress interaction: F1,66 = 9.452, p = .0031). There was also a significant Time Period X Extinction interaction (F1,66 = 5.966, p = .0173), reflecting that the effect of shock was slightly larger in the extinguished groups (Effect of Time Period: p = .0040, Bonferroni adjusted) than the non-extinguished groups (Effect of Time Period: p = .3902). In the Context C test session 24h after conditioning (Figure 2G), freezing was higher in stressed than nonstressed mice (Effect of Stress: F1,1 = 16.508, p = .0001) and there were no significant effects of extinction training.
In summary, we found that prior footshock stress reduces spontaneous exploration in the open field, increases unconditioned freezing to a novel tone, and potentiates contextual fear conditioning. Extinction training after footshock stress restored normal exploratory behavior but failed to attenuate stress effects on unconditioned fear and contextual fear conditioning.
Experiment 2: 10 Sessions of Extinction Training
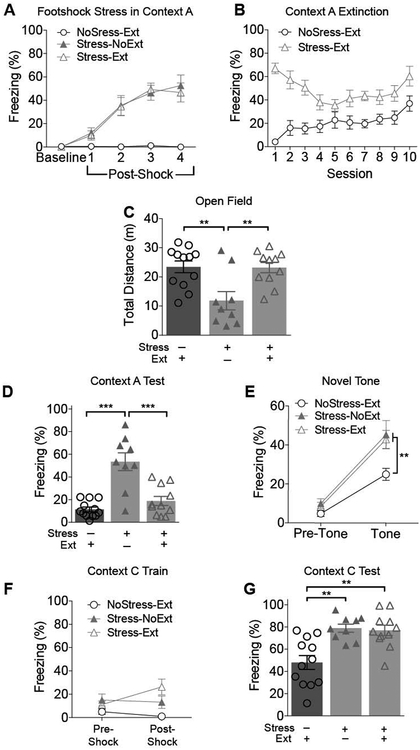
Although context fear extinction training failed to alleviate all of the effects of footshock stress in Experiment 1, there is the possibility that more extensive extinction training would fully restore normal fear and anxiety-like behavior. Therefore, in Experiment 2, we asked whether extended extinction training would alleviate all the effects of footshock stress on tests of conditioned and unconditioned fear. The sequence of behavioral tests was identical to that of Experiment 1, except mice received 10 sessions of extinction training in Context A rather than 5 sessions. In addition, because groups NoStress-NoExt and NoStress-Ext performed similarly in Experiment 1, only the NoStress-Ext Group was included in this experiment. Thus, there were three experimental groups: Stress-NoExt (n=5 female, 4 male), Stress-Ext (n=6 female, 5 male), and NoStress-Ext (n=7 female, 5 male).
In the footshock stress session, freezing behavior increased as a function of shock (Figure 3A) and remained low in mice that did not receive footshock. Extinction data (Figure 3B) were analyzed using Group X Session RM-ANOVA, which yielded a significant interaction effect: F9, 135 = 4.750, p < .0001. Freezing declined from session 1 to 5 (t135 = 4.815, p < .0001, Bonferroni-corrected) and then increased (Session 5 v 10: t135 = 2.032, p = .0020), presumably owing to increased immobility (habituation of exploration) with extended context exposure. In Group NoStress-Ext, freezing also increased modestly over sessions (Session 1 v 10: t135 = 4.709, p < .0001). Due to camera malfunction 6 mice (2 in Group Stress-Ext and 4 in Group NoStress-Ext) were excluded from the extinction session analysis (but these mice were included in all other tests and analyses).
Figure 3.
Results of Experiment 2 (10 sessions of extinction training). (A) During the footshock stress session, freezing behavior increased as a function of the 4 shock presentations. (B) Freezing behavior during Context A extinction training. (C) Prior footshock stress suppressed open field exploration. This effect was completely reversed by extinction training. (D) Freezing behavior during the test session in the stress context (Context A). Extinction training reduced freezing behavior in stressed mice to the levels of mice that did not receive footshock stress. (E) Prior footshock stress potentiated unconditioned fear of a novel tone. This effect of stress was not attenuated by extinction training. (F) Prior footshock stress increased freezing during contextual fear conditioning in a novel context (Context C). There was no significant effect of extinction training. (G) In the Context C test session, context-elicited fear was enhanced in mice that received prior footshock stress. This effect was not reduced by extinction training. NoStress-Ext (n=12); Stress-NoExt (n=9); Stress-Ext (n=11). *p<0.05, **p<0.01, ***p<0.001
In the open field (Figure 3C), exploration was reduced in group Stress-NoExt as compared to both groups NoStress-Ext and Stress-Ext (Main effect of Group: F2 = 7.843, p = .0019), confirming that footshock stress suppresses open field exploration and that this suppression is alleviated by extinction.
After completion of extinction training, all mice received a test of contextual fear in Context A (Figure 3D). Context-elicited fear was significantly higher in Group Stress-NoExt than groups Stress-Ext and NoStress-Ext (Main effect of Group: F2 = 31.161, p< .0001; see also Suppl. Table 2). Freezing levels of Groups Stress-Ext and NoStress-Ext did not differ (Tukey HSD test: p = .4811), indicating that extinction training reduced conditioned fear to the level of unshocked controls.
In contrast to the open field results, extended extinction training failed to alleviate the effects of footshock stress in the tests of unconditioned and conditioned fear. Unconditioned tone-elicited fear (Figure 3E) was enhanced in groups Stress-NoExt and Stress-Ext as compared to group NoStress-Ext (Group X Time Period Interaction: F2,29 = 4.794, p = .0159), indicating that extended extinction training failed to reverse the effects of footshock stress on unconditioned fear. During the conditioning session in Context C (Figure 3F), baseline (pre-shock) freezing was low in all groups and increased in the post-shock period. RM-ANOVA showed a significant Time Period X Group interaction (F2,29 =3.697, p = .0372). Post hoc tests confirmed that the groups did not differ during the pre-shock period. During the post-shock period freezing of group Stress-Ext exceeded that of group NoStress-Ext (p = .0001), but the other comparisons did not reach significance. In the test of context-elicited fear 24h after conditioning (Figure 3G), groups Stress-NoExt and Stress-Ext exhibited similarly high levels of freezing that exceeded that of group NoStress-Ext (Main effect of Group: F2 = 11.335, p = .0002).
In summary, the results of Experiment 2 replicate the findings of Experiment 1. Footshock stress reduced spontaneous exploration in the open field, increased unconditioned freezing to a novel tone, and potentiated contextual fear conditioning in a novel context. Extensive extinction training after footshock stress restored normal exploratory behavior in the open field but failed to alleviate stress effects on unconditioned fear and contextual fear conditioning.
Sex differences in the effects of footshock stress
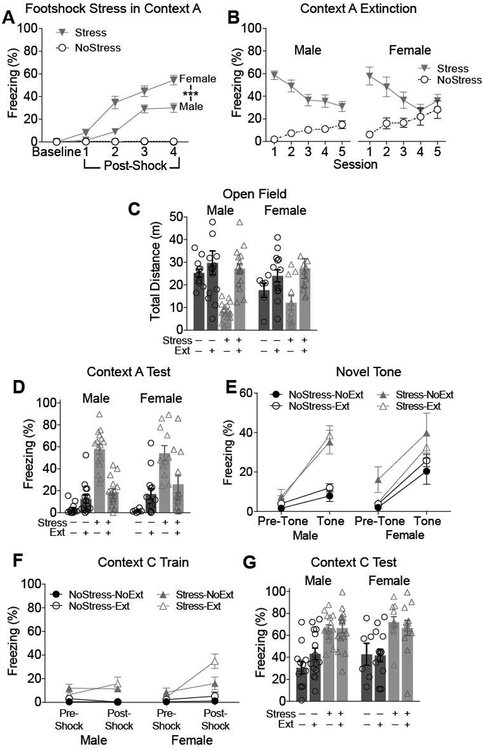
There are prominent sex differences in the behavioral and neural responses to stress and in the incidence of stress-related disorders such as PTSD (Tolin & Foa, 2006). Although male and female mice were used in Experiments 1 and 2, the individual studies were not powered to detect sex differences. To evaluate potential sex differences, the data from Experiments 1 and 2 were aggregated and re-analyzed with Sex as a factor.
During footshock stress (Figure 4A), shocked females showed elevated freezing compared to males in the post-shock periods (Sex X Stress X Trial Interaction: F4,95 = 7.311, p <.0001). However, in the extinction sessions (Figure 4B), there were no significant sex differences (Suppl. Table 3). Although females displayed a higher acute response to footshock stress than males, the sexes did not differ in later expression or extinction of learned fear.
Figure 4.
Analysis of sex effects using data aggregated across Experiments 1 and 2. (A) Freezing was higher in females than males during the footshock stress session in Context A. (B) During extinction training, there were no significant effects of sex. (C) Prior footshock stress suppressed open field exploration in both males and females. There were no significant effects of sex. (D) During the test session in Context A, there were no significant effects of sex. (E) In the test of unconditioned tone-elicited fear, unstressed females exhibited more tone-elicited fear than unstressed males, but there were no sex differences among stressed mice. (F) During contextual fear conditioning in Context C, females froze more than males during the post-shock period regardless of stress or extinction status. (G) No effects of sex during the Context C test session. NoStress-NoExt (n=12 males, 6 females); NoStress-Ext (n=15 males, 14 females); Stress-NoExt (n=16 males, 11 females); Stress-Ext (n= 16 males, 12 females).
In the open field, there were no effects of sex (Figure 4C). In the test of conditioned fear in Context A (Figure 4D), there were no sex differences, consistent with performance during the extinction sessions.
In contrast, effects of sex were observed in the assays of unconditioned fear and fear conditioning. In the test of unconditioned tone-elicited fear (Figure 4E), there was a significant Sex X Time Period X Stress interaction (F1,94 = 7.677, p =.0067) reflecting that, among mice that were not previously stressed, females exhibited more tone-elicited freezing than males (Tukey HSD =14.021, p < .0001). There were no sex differences among stressed mice or during the pre-tone period. This result indicates that unstressed females exhibit a stronger unconditioned tone fear response than that of unstressed males, but the sex difference is abolished in stressed mice.
During single-shock contextual fear conditioning in Context C (Figure 4F), females exhibited more freezing than males during the post-shock period regardless of stress or extinction status (Time Period X Sex interaction: F1,94 = 7.908, p =.0060). Pairwise comparisons confirmed that, regardless of prior stress or extinction, female freezing exceeded male freezing during the post-shock period (Tukey HSD, p = .0200) but not during the pre-shock period (p = .3398). During the text of context-elicited fear 24h after training (Figure 4G), there were no sex differences (Suppl. Table 3). In summary, the sex analysis demonstrates that females displayed more freezing than males in response to acute aversive stimulation, but the long-term effects of footshock stress were otherwise similar between sexes.
Discussion
Our experiments establish a SEFL protocol in male and female 129s6 mice. In this protocol, footshock stress caused three main effects: it suppressed of open field exploration, potentiated unconditioned fear, and enhanced contextual fear conditioning in a novel context. Extinguishing contextual fear of the original stress context restored normal open field exploration but failed to ameliorate stress effects on unconditioned fear and fear conditioning. Because open field exploration was normalized by context extinction, we interpret the open field effect as representing generalization of fear/anxiety generated by the stress context or related cues (e.g., handling cues). In contrast, the stress-induced enhancements of unconditioned fear and fear conditioning were insensitive to context fear extinction, suggesting that these stress effects represent nonassociative sensitization, similar to what has been demonstrated in rat studies. In summary, our results establish a SEFL paradigm that can be used to evaluate associative and nonassociative effects of footshock stress in mice.
PTSD includes symptoms with both associative and nonassociative characteristics (Friedman et al., 2010). Associative-like symptoms are those that involve memories of the original trauma, such as exaggerated physiologic and emotional responses to stimuli resembling those present during the trauma, intrusive “flashback” memories of the trauma, and avoidance of trauma reminders. Nonassociative-like symptoms are those without a direct link to trauma-related stimuli or memories. These include persistent fear, irritability, hypervigilance and exaggerated startle response. Our results and similar findings (Golub et al., 2009; Rau & Fanselow, 2009) suggest that extinction training and its clinical analog, exposure therapy, will be successful in reducing the severity of associative symptoms but less effective in treating nonassociative symptoms. To our knowledge, the comparative effectiveness of exposure therapy with respect to these different symptom categories has not been evaluated in PTSD patients. However, consistent with our prediction, clinical studies indicate that exposure therapy, while effective at reducing symptom severity, does not abolish all PTSD symptoms (Bradley et al., 2005).
Our finding that extinguishing fear of the stress context fails to alleviate fear sensitization leads us to conclude that fear sensitization and Pavlovian fear conditioning are distinct processes. This conclusion is supported by a number of other studies. Siegmund and Wotjak (Siegmund & Wotjak, 2007b) demonstrated that pharmacologic inhibition of hippocampus during footshock stress reduces fear conditioning to the stress context but does not prevent the stress from enhancing other unconditioned fear responses. When footshock stress is administered to rats too young to acquire Pavlovian contextual fear, the rats still exhibit fear sensitization when tested as adults (Poulos et al., 2014). Similarly, human patients with amnesia of an acutely traumatic event can still exhibit symptoms of PTSD (Layton, Krikorian, Dori, Martin, & Wardi, 2006). We are aware of only one study showing that fear sensitization requires Pavlovian conditioned fear. Golub et al. (2009) showed that extinction of contextual fear one day after footshock stress abolishes stress-enhanced acoustic startle. Interestingly, in the same study, when extinction was conducted 25 d after stress it failed to attenuate stress-enhanced startle. In the current study, extinction training beginning one day after footshock stress failed to attenuate fear sensitization as measured via freezing behavior. The contrast with Golub et al. (2009) suggests that startle sensitization and freezing sensitization may have different underlying mechanisms.
Although the neural mechanisms of fear sensitization are not yet well understood, there is mounting evidence that sensitization and Pavlovian fear conditioning have different neural mechanisms. Pharmacological blockade of N-methyl-D-aspartate (NMDA) receptors during footshock stress prevents acquisition of associative fear to the stress context but does not prevent SEFL (Rau et al., 2005). Perusini et al. (2015) showed that blockade of corticosterone (CORT) production via the drug metyrapone prevents induction of both associative fear conditioning and SEFL. Nevertheless, supplementing metyrapone-treated rats with CORT restored SEFL but not associative fear conditioning, suggesting that fear sensitization and associative fear conditioning require different amounts or timing of CORT. Finally, SEFL was associated with an increase in amygdala expression of the GluA1 α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor (AMPAR) subunit (Perusini et al., 2015). Increased expression of GluR1 is also believed to mediate the increased abundance of calcium-permeable AMPARs that is associated with acquisition of Pavlovian fear memories (Humeau et al., 2007; Kessels & Malinow, 2009). However, the increase in calcium-permeable AMPARs caused by Pavlovian fear conditioning dissipates within 1 week after conditioning (Clem & Huganir, 2010). Because the increase in GluA1 expression caused by SEFL training lasts for at least 2 weeks, Perusini et al (2015) hypothesized that fear sensitization involves a more enduring change in AMPA subunit composition than does associative fear conditioning. Further studies are needed to clarify how the neural mechanisms of associative and nonassociative fear diverge.
The present study revealed some sex differences in stress responses that may have clinical relevance. The incidence of PTSD is greater in women than men, even among people who experience the same type of traumatic event (Tolin & Foa, 2006), suggesting that the sex difference is not caused strictly by differences in trauma exposure. There are also sex differences in PTSD symptomology (Bangasser, Eck, Telenson, & Salvatore, 2018). Among people diagnosed with PTSD, women have more pronounced sleep disturbances than men (Kobayashi & Mellman, 2012), which has been interpreted as reflecting more pronounced hyperarousal in women (Bangasser et al., 2018). Even among healthy people, women display stronger physiological responses to strong negative stimuli than men (Bangasser et al., 2018). Consistent with a study of SEFL in rats (Poulos et al., 2015), we did not observe sex differences in the effects of stress on exploratory behavior, unconditioned fear or fear conditioning. We did, however, find that female mice displayed stronger responses than males to acute stressors. During the footshock stress session, females exhibited more freezing than males, consistent with another study (Horst, Carobrez, van der Mark, de Kloet, & Oitzl, 2012). Among non-stressed mice, females displayed stronger freezing than males to the novel tone. And during single-shock contextual conditioning, females displayed higher post-shock freezing than males. The increased freezing of female mice during the fear conditioning sessions did not persist in the shock-free fear recall test sessions, suggesting that the sexes differ in the acute response to aversive stimulation but not in the long-term associative and nonassociative memories generated by this stimulation.
One limitation of the current studies is that the tests of exploration and fear occurred in sequence without counterbalancing of the test order. As a result, we cannot exclude the possibility that context extinction training had the strongest effect in the open field test because this test occurred closest in time to extinction training. Because conditioned fear spontaneously recovers over time after extinction training, it is possible that Context A fear had recovered somewhat by the time of the other fear-based assays. There are two reasons to discount this explanation, however. First, the duration between tests was quite brief compared to the time needed to observe robust spontaneous recovery in our hands. In the current studies, all tests occurred within 5 days after the end of extinction training. In Lacagnina et al. (2019), we observed no spontaneous recovery over this time period. Second, spontaneous recovery usually involves only a partial recovery of the conditioned response (Rescorla, 2004). Even if some spontaneous recovery had occurred, the extinguished mice likely would have had weaker contextual fear than non-extinguished mice.
In summary, we established a mouse model of SEFL that produces robust changes in exploratory behavior, unconditioned fear, and fear learning. Extinguishing conditioned fear of the stress context restores normal exploratory behavior but does ameliorate stress-enhanced fear and fear learning. Consequently, we conclude that acute traumatic stress affects behavior through both associative and nonassociative mechanisms, each likely to be mediated by distinct physiological processes.
Supplementary Material
Acknowledgements
Supported by NIH grant nos. R01 MH102595 and R01 MH117426.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bangasser DA, Eck SR, Telenson AM, & Salvatore M (2018). Sex differences in stress regulation of arousal and cognition. Physiology & Behavior, 187, 42–50. 10.1016/j.physbeh.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, & Westen D (2005). A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry, 162(2), 214–227. 10.1176/appi.ajp.162.2.214 [DOI] [PubMed] [Google Scholar]
- Clem RL, & Huganir RL (2010). Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science (New York, NY), 330(6007), 1108–1112. 10.1126/science.1195298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, Resick PA, Bryant RA, & Brewin CR (2010). Considering PTSD for DSM-5. Depression and Anxiety, 28(9), 750–769. [DOI] [PubMed] [Google Scholar]
- Golub Y, Mauch CP, Dahlhoff M, & Wotjak CT (2009). Consequences of extinction training on associative and non-associative fear in a mouse model of Posttraumatic Stress Disorder (PTSD). Behavioural Brain Research, 205(2), 544–549. 10.1016/j.bbr.2009.08.019 [DOI] [PubMed] [Google Scholar]
- Horst, ter JP, Carobrez AP, van der Mark MH, de Kloet ER, & Oitzl MS (2012). Sex differences in fear memory and extinction of mice with forebrain-specific disruption of the mineralocorticoid receptor. European Journal of Neuroscience, 36(8), 3096–3102. 10.1111/j.1460-9568.2012.08237.x [DOI] [PubMed] [Google Scholar]
- Huckleberry KA, Ferguson LB, & Drew MR (2016). Behavioral mechanisms of context fear generalization in mice. Learning & Memory (Cold Spring Harbor, NY), 23(12), 703–709. 10.1101/lm.042374.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Reisel D, Johnson AW, Borchardt T, Jensen V, Gebhardt C, et al. (2007). A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning. Journal of Neuroscience, 27(41), 10947–10956. 10.1523/JNEUROSCI.2603-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, & Wotjak CT (2004). Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learning & Memory, 11(6), 770–786. 10.1111/j.1460-9568.2012.08237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, & Malinow R (2009). Synaptic AMPA Receptor Plasticity and Behavior. Neuron, 61(3), 340–350. 10.1016/j.neuron.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, & Mellman TA (2012). Gender differences in sleep during the aftermath of trauma and the development of posttraumatic stress disorder. Behavioral Sleep Medicine, 10(3), 180–190. 10.1080/15402002.2011.654296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell, 131(2), 391–404. 10.1016/j.cell.2007.09.018 [DOI] [PubMed] [Google Scholar]
- Lacagnina AF, Brockway ET, Crovetti CR, Shue F, McCarty MJ, Sattler KP, et al. (2019). Distinct hippocampal engrams control extinction and relapse of fear memory. Nature Neuroscience, 22(5), 753–761. 10.1038/s41593-019-0361-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton BS, Krikorian R, Dori G, Martin GA, & Wardi K (2006). Posttraumatic stress disorder with amnesia following asphyxiation. Annals of the New York Academy of Sciences, 1071(1), 488–490. 10.1196/annals.1364.048 [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ (1974). The psychology of animal learning. London ; New York: : Academic Press. [Google Scholar]
- Mineur YS, Belzung C, & Crusio WE (2006). Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behavioural Brain Research, 175(1), 43–50. 10.1016/j.bbr.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Nollet M, Le Guisquet A-M, & Belzung C (2013). Models of depression: unpredictable chronic mild stress in mice. Current Protocols in Pharmacology, Chapter 5(1), Unit 5.65–5.65.17. 10.1002/0471141755.ph0565s61 [DOI] [PubMed] [Google Scholar]
- Perusini JN, Meyer EM, Long VA, Rau V, Nocera N, Avershal J, et al. (2015). Induction and Expression of Fear Sensitization Caused by Acute Traumatic Stress. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 41(1), 45–57. 10.1038/npp.2015.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzimenti CL, Navis TM, & Lattal KM (2017). Persistent effects of acute stress on fear and drug-seeking in a novel model of the comorbidity between post-traumatic stress disorder and addiction. Learning & Memory (Cold Spring Harbor, NY), 24(9), 422–431. 10.1101/lm.044164.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Reger M, Mehta N, Zhuravka I, Sterlace SS, Gannam C, et al. (2014). Amnesia for Early Life Stress Does Not Preclude the Adult Development of Posttraumatic Stress Disorder Symptoms in Rats. Biological Psychiatry, 76(4), 306–314. 10.1016/j.biopsych.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Zhuravka I, Long V, Gannam C, & Fanselow M (2015). Sensitization of fear learning to mild unconditional stimuli in male and female rats. Behavioral Neuroscience, 129(1), 62–67. 10.1037/bne0000033 [DOI] [PubMed] [Google Scholar]
- Rau V, & Fanselow MS (2009). Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress (Amsterdam, Netherlands), 12(2), 125–133. 10.1080/10253890802137320 [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, & Fanselow MS (2005). Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews, 29(8), 1207–1223. 10.1016/j.neubiorev.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Rescorla RA (2004). Spontaneous Recovery. Learning & Memory, 11(5), 501–509. 10.1101/lm.77504 [DOI] [PubMed] [Google Scholar]
- Seo D-O, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, & Drew MR (2015). Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms. Journal of Neuroscience, 35(32), 11330–11345. 10.1523/JNEUROSCI.0483-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund A, & Wotjak CT (2007a). A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. Journal of Psychiatric Research, 41(10), 848–860. 10.1016/j.jpsychires.2006.07.017 [DOI] [PubMed] [Google Scholar]
- Siegmund A, & Wotjak CT (2007b). Hyperarousal does not depend on trauma-related contextual memory in an animal model of Posttraumatic Stress Disorder. Physiology & Behavior, 90(1), 103–107. 10.1016/j.physbeh.2006.08.032 [DOI] [PubMed] [Google Scholar]
- Sillivan SE, Jamieson S, Nijs L, Jones M, Snijders C, Klengel T, et al. (2019). MicroRNA regulation of persistent stress-enhanced memory. Molecular Psychiatry, 62, 1–12. 10.1038/s41380-019-0432-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillivan SE, Joseph NF, Jamieson S, King ML, Chévere-Torres I, Fuentes I, et al. (2017). Susceptibility and Resilience to Posttraumatic Stress Disorder-like Behaviors in Inbred Mice. Biological Psychiatry, 82(12), 924–933. 10.1016/j.biopsych.2017.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, & Foa EB (2006). Sex differences in trauma and posttraumatic stress disorder: A quantitative review of 25 years of research. Psychological Bulletin, 132(6), 959–992. 10.1037/0033-2909.132.6.959 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.