Abstract
Background:
Endovascular aortic aneurysm repair (EVAR), left ventricular assist device (LVAD), and transcatheter aortic valve replacement (TAVR) are expensive cardiovascular technologies with potential to benefit large numbers of patients. There are few population-based studies comparing utilization between countries. Our objective was to compare patient characteristics and utilization patterns of EVAR, LVAD, and TAVR in Ontario, Canada and New York State, United States (US).
Methods and Results:
We performed a retrospective cohort study using administrative data to identify all adults who received EVAR, LVAD, or TAVR in Ontario and New York between 2012–2015. We compared socio-demographics of EVAR, LVAD, and TAVR recipients in Ontario and New York. We compared standardized utilization rates between jurisdictions for each procedure. We identified 3,295 EVAR recipients from Ontario and 6,236 from New York (mean age 74.6 vs. 74.5 years; P=.61): 136 LVAD recipients from Ontario and 686 from New York (age 57.4 vs. 57.7 years; P=.80): 1,708 TAVR recipients from Ontario and 4,838 from New York (age 83.1 vs. 83.1; P=1.0). A significantly smaller percentage of EVAR and TAVR recipients in Ontario were female compared to New York (EVAR 15.8% vs 22.1% female; P<.001)(TAVR 45.9% vs 51.8%; P<.001), but for LVAD the percentage female was similar (21.3% vs. 20.8%; P=.99). Utilization was significantly higher in New York for all procedures: EVAR (12.8 procedures per-100,000 adults per-year in Ontario, 20.2 in New York; P<.001); LVAD (0.3 in Ontario vs. 1.3 in New York; P<.001); TAVR (6.6 in Ontario, 14.3 in New York; P<.001). Higher utilization of EVAR and TAVR in New York relative to Ontario increased substantially with increasing age.
Conclusions:
We observed significantly higher utilization of EVAR, LVAD, and TAVR in New York compared to Ontario. Our results highlight important differences in how two different countries are using advanced cardiovascular therapies.
Introduction
Over the past three decades endovascular aortic aneurysm repair (EVAR), left ventricular assist device (LVAD), and transcatheter aortic valve replacement (TAVR) have evolved from unproven innovations to established therapies for selected patients with cardiovascular conditions based upon high-profile randomized trials.1–3 Subsequent follow-up studies and systematic reviews have added nuance about effectiveness and expanded indications.4–6 While insurance coverage of and access to EVAR, LVAD, and TAVR may differ among countries, the underlying clinical indications and practice guidelines are generally similar.7, 8
Even with a similar body of underlying evidence, regulators and payers in different jurisdictions may approach neoteric therapeutics differently.9, 10 In many ways the idea that different countries (or even regions within countries) may offer and pay for differential access to various therapeutics makes sense and should reflect local preferences and values; Papanicolas and Jha articulate these issues very clearly in a recent JAMA perspective.11
A limited body of literature has described utilization of EVAR,12 LVAD,13, 14 and TAVR15 within single countries, but very few studies have compared utilization between countries.16 There is growing interest in international comparative health systems research as a mechanism for understanding how between-country differences in healthcare policy impact spending, utilization, and patient outcomes.11, 17 However there are very few international comparative cardiovascular studies and most focus on older technologies.18, 19 Comparing utilization of EVAR, LVAD, and TAVR between countries can help to provide insight into how national values, funding, and policy can effect patients’ access to costly therapies.11
We used contemporary administrative health data from Ontario (Canada) and New York State (US) to examine differences in patient characteristics, utilization, and clinical outcomes for EVAR, LVAD, and TAVR. These procedures were selected because they are relatively new technologies with established effectiveness, but also evolving clinical indications. We compared Ontario with New York State due to their vastly different health care systems, but geographic proximity and similarly populations with respect to racial/ethnic diversity.19, 20 We hypothesized a priori that there would be significantly higher overall utilization of each procedure in New York compared to Ontario but that patterns of utilization would differ in key patient subgroups. In particular, building upon prior studies by Gorey et al,21, 22 we expected that we would find a utilization gradient in New York (higher utilization for residents of high income neighborhoods, lower utilization for low income neighborhoods) that would be less apparent in Ontario.
Methods
Data
We used administrative data from the most populous Canadian province (Ontario: population 14.3 million) and a large US state (New York: population 19.8 million), building on prior international comparative work.19, 23 Ontario and New York share a common border, are diverse, and have an extremely large city (Toronto and New York City), and significant rural areas.
Our primary data source for Ontario was the 2011–2015 Discharge Abstract Database (DAD) obtained through ICES. The administrative records obtained from CIHI-DAD provided information on all hospitalizations paid for by the Ontario Health Insurance Plan (OHIP); OHIP provides health insurance to all legal residents of Ontario (~99% of the population) and virtually 100% of inpatient hospitalizations. Ontario’s DAD provides information regarding demographic characteristics (age, sex), primary and secondary diagnoses using International Classification of Diseases Version 10 (ICD-10) codes, procedures using Canadian Classification of Health Interventions (CCI) codes, discharge disposition (e.g., died-in-hospital, home, transfer to another acute-care hospital), a unique patient identifier and unique hospital identifier. Comorbid conditions coded on the index hospital stay were identified using the Quan adaptation of the Elixhauser coding scheme.24
For New York we used data from the 2011–2015 State Inpatient Database (SID).23, 25 The SID contains administrative data for all patients admitted to non-governmental acute care hospitals. Data elements for each admission include patient demographics, primary and secondary diagnosis and procedures (coded using ICD9-CM codes), discharge disposition, patient identifier, and hospital identifier. Comorbid conditions for the index hospital stay were captured using algorithms developed by Elixhauser et al.26
Estimates of the New York population were obtained from US Census Data; estimates of the Ontario population were obtained from Canadian Census Data. We linked the New York data to the American Hospital Association annual survey to ascertain information regarding hospital teaching status and bed size. We linked the Ontario CIHI-DAD to information from the Ontario Ministry of Health and Long-Term Care for hospital-level data.
Some of the NY SID data supporting our findings may be available from the corresponding author by request (peter.cram@uhn.ca); the Ontario ICES data can not be shared, but some of the supporting analyses may be available by request. Statistical code may be available by request.
Study cohorts
We used CCI codes in Ontario and ICD9-CM codes in New York to identify adults aged 18–104 years who received each of the above procedures between January 1, 2012 and September 30, 2015 (see Supplemental Table 1 for list of CCI and ICD9-CM codes).27–30 For TAVR and EVAR, we excluded patients < 40 years of age as aortic stenosis and AAA are rare in that age group, while for LVAD younger recipients are common and thus were included. We also excluded patients who resided outside of Ontario and New York, and those for which EVAR, LVAD, or TAVR was not listed in the primary procedure field. We also excluded patients who received their procedures in hospitals with implausibly low procedure volumes (<1 procedure per-year). We used a 365-day lookback period (January 1, 2011-December 31, 2011) to exclude patients who were undergoing a repeat procedure (e.g., LVAD followed by LVAD). We allowed for patients to undergo multiple different procedures (e.g., LVAD followed by EVAR) so long as the 2 different procedures occurred during separate hospitalizations. Our study protocol was developed before initiation of any analyses and is available online through Open Science at https://osf.io/brxd3/.
Statistical analyses
First, at the patient level, we compared the characteristics of patients who underwent any of our 3 procedures (EVAR, LVAD, TAVR) in Ontario and New York including demographics and comorbid conditions using bivariate methods. Postal code of residence for each patient was linked to census-level neighborhood income; all postal codes in Ontario and New York were then stratified into quintiles with respect to income (quintile 1= lowest income; quintile 5= highest income). For each procedure we compared the proportion of recipients who resided in the lowest income quintiles (quintile 1 and 2) and the highest income quintiles (4 and 5) in Ontario and New York. Second, we compared the percentage of all acute care hospitals in Ontario and New York that performed each of our 3 procedures and annual hospital volumes.
Third we compared the per-capita procedure rates (procedures per-100,000 per-year) for adults in Ontario and New York for EVAR, LVAD, and TAVR. The numerator was the total number of procedures performed and the denominator was the number of adults age ≥ 40-years (age ≥18 for LVAD) in each jurisdiction in 2014. We calculated age and sex standardized utilization rates (Ontario as the reference) using direct standardization. We then conducted stratified analyses by decade of age and sex. We examined per-capita age and sex standardized procedure rates stratified by neighborhood income quintile in Ontario and New York; for these analyses the numerator was number of procedures performed on patients residing in each income quintile while the denominator was the number of adults residing in each income quintile. Fourth we evaluated changes in volume and per-capita utilization for each year to examine whether there might be changes in utilization over our-admittedly brief- study period.
Fifth, we compared unadjusted and adjusted outcomes for recipients of EVAR, LVAD, and TAVR in Ontario and New York; outcomes included hospital length of stay (LOS), in-hospital mortality, and hospital readmission within 90-days of discharge among those who survived to discharge. We used generalized estimating equations to calculate standardized estimates for LOS, readmission within 90-days of discharge, and in-hospital mortality adjusting for age, sex, and hospital procedure volume. We conducted additional analyses looking at mortality within 7-days of the index hospital procedure using 3 different models: Model 1 adjusted for volume only; Model 2 adjusted for comorbidities only; Model 3 adjusted for hospital procedure volume plus comorbidities.
This analysis was approved by the Research Ethics Board at University Health Network, Toronto. Analysis of Ontario data was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board. Analyses were performed using SAS (Cary, North Carolina) or R statistical software packages.
Results
For EVAR, we identified 3,295 recipients in Ontario and 6,239 recipients in New York between 2012–2015 (Supplemental Figures 1 and 2). The corresponding numbers for LVAD were 136 in Ontario and 686 in New York and for TAVR were 1,708 in Ontario and 4,838 in New York (Table 1). Age of EVAR, LVAD, and TAVR recipients was similar in Ontario and New York. The percentage of LVAD recipients who were female was similar in Ontario and New York, but the percentage of EVAR recipients who were female was significantly lower in Ontario than New York (15.8% vs 22.1%; P<.001) and likewise for TAVR (45.9% vs 51.8%; P<.001). Patients receiving both EVAR and TAVR in Ontario were significantly more likely to reside in neighborhoods in the lowest quintiles of income (quintiles 1 and 2) compared to patients in New York (Table 1); alternatively, patients receiving EVAR and TAVR in Ontario were significantly less likely to reside in neighborhoods in the highest quintiles of income (quintiles 4 and 5). The prevalence of comorbidities was significantly lower in Ontario as compared to New York for all 3 conditions. A smaller percentage of hospitals in Ontario than New York performed EVAR, LVAD, and TAVR (Table 2), but was only statistically significant for EVAR. Examination of median volumes and inter-quartile ranges (Table 2) demonstrated a larger number of low-volume hospitals in New York, particularly for EVAR where 50% of hospitals performed 11-or-fewer procedures per-year.
Table 1:
Characteristics of patients who underwent EVAR, LVAD, and TAVR in Ontario and New York in 2012–2015*
| EVAR | LVAD | TAVR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ontario (N=3295) |
NY (N=6236) |
P-value | Ontario (N=136) |
NY (N=686) |
P-value | Ontario (N=1708) |
NY (N=4838) |
P-value | |
| Age, mean (sd) | 74.6 (9.1) | 74.5 (9.1) | 0.61 | 57.4 (12.7) | 57.7 (12.7) | 0.802 | 83.1 (7.7) | 83.1 (7.7) | 1 |
| Female, N(%) | 522 (15.8) | 1379 (22.1) | <.001 | 29 (21.3) | 143 (20.8) | 0.992 | 784 (45.9) | 2505 (51.8) | <.001 |
| Income Quintile 1 or 2 | 1300 (39.5) | 2195 (35.2) | <.001 | 58 (42.6) | 262 (38.2) | 0.380 | 625 (36.6) | 1060 (21.9) | <.001 |
| Income Quintile 3 | 653 (19.8) | 1137 (18.2) | 0.063 |
27 (19.9) | 112 (16.3) | 0.380 | 348 (20.4) | 713 (14.7) | <.001 |
| Income Quintile 4 or 5 | 1337 (40.6) | 2854 (45.8) | <.001 | 51 (37.5) | 305 (44.5) | 0.161 | 726 (42.5) | 3040 (62.8) | <.001 |
| Comorbid conditions | |||||||||
| Congestive heart failure | 82 (2.5) | 695 (11.1) | <.001 | 128 (94.1) | 672 (98.0) | 0.025 | 543 (31.8) | 3922 (81.1) | <.001 |
| Depression | 13 (0.4) | 420 (6.7) | <.001 | 11 (8.1) | 50 (7.3) | 0.884 | 9 (0.5) | 271 (5.6) | <.001 |
| Hypertension with complications | 10 (0.3) | 958 (15.4) | <.001 | 0 (0.0) | 174 (25.4) | <.001 | 9 (0.5) | 1399 (28.8) | <.001 |
| Diabetes without complications | 206 (6.3) | 1128 (18.1) | <.001 | SC† | 129 (18.8) | <.001 | 61 (3.6) | 1202 (24.8) | <.001 |
| COPD | 310 (9.4) | 1916 (30.7) | <.001 | 12 (8.8) | 277 (40.4) | <.001 | 156 (9.1) | 2289 (47.3) | <.001 |
| Renal Failure | 133 (4.0) | 998 (16.0) | <.001 | 17 (12.5) | 243 (35.4) | <.001 | 182 (10.7) | 1623 (33.5) | <.001 |
Each patient counted only once in each cohort (so a patient who underwent both TAVR and LVAD could appear once for each)
SC = small cells, cells <6 cannot be identified due to ICES privacy regulations
Table 2:
Characteristics of hospitals that performed EVAR, LVAD, and TAVR in Ontario and New York*
| EVAR | LVAD | TAVR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ON (N=165) | NY (N=224) | P-value | Ontario (N=165) | NY (N=224) | P-value | Ontario (N=165) | NY (N=224) | P-value | |
| Hospitals performing procedure, number (%) | 15 (9.1) | 86 (38.4) | <.001 | 3 (1.8) | 11 (4.9) | 0.179 | 10 (6.1) | 23 (10.3) | .198 |
| Annual procedural volume, mean (SD) | 58.4 (46.9) | 19.7 (25.1) | .007 | 13.0 (7.8) | 16.9 (14.9) | 0.559 | 58.7 (27.9) | 56.3 (48.5) | .859 |
| Annual procedural volume, median (Inter-quartile range) | 41.0 (18.0–93.0) | 10.9 (3.3 – 23.9) | NA | 9.0 (8.0–22.0) | 9.1 (6.9 – 28.0) | NA | 65.5 (32.0–76.0) | 39.2 (24.7 – 74.8) | NA |
| Bed number, mean (SD) | 406.1 (193.4) | 536.0 (430.7) | .064 | 591.3 (393.5) | 1068.7 (700.7) | 0.175 | 462.4 (218.7) | 871.7 (580.5) | .006 |
| Major teaching*, number (%) | 8 (53.3) | 29 (34.5) | .244 | 3 (100.0) | 10 (90.9) | 1 | 8 (80.0) | 18 (78.3) | 1 |
Denominator is total number of hospitals performing the procedure of interest in the jurisdiction
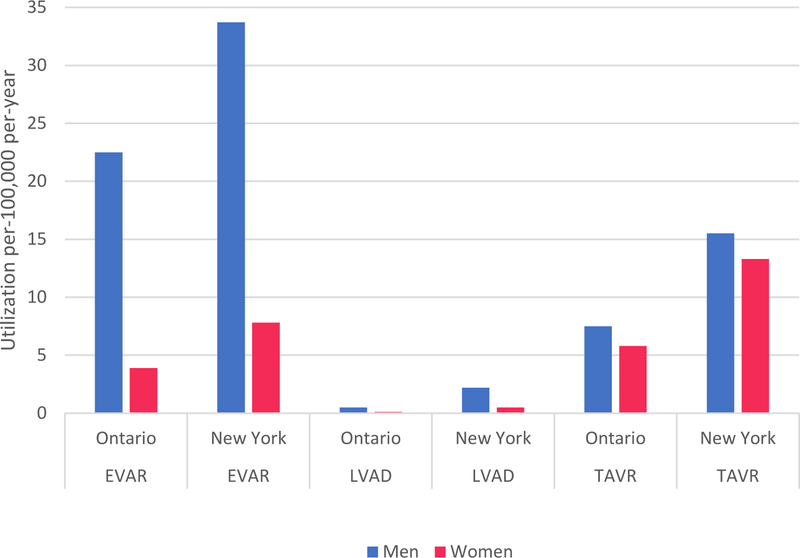
Table 3 includes per-capita utilization of all 3 procedures, standardized for age and sex (per-100,000 per-year). Utilization was significantly greater in New York than Ontario for EVAR (20.1 vs. 12.8; P <0.001), LVAD (1.3 vs. 0.3; P <.001) and TAVR (14.3 vs. 6.8; P <0.001). Higher utilization in New York compared to Ontario was observed in in both men and women (Figure) and was particularly notable in older age groups for EVAR and TAVR (Supplemental Figure 3). Volume and utilization for EVAR in New York and Ontario and LVAD in New York were quite stable between 2012–2015 (Supplemental Tables 2–3 and Supplemental Figure 4). Alternatively, volumes and utilization of LVAD in Ontario increased slightly, while volumes and utilization of TAVR in New York and Ontario increased substantially.
Table 3:
| Ontario | New York | EVAR Utilization | LVAD Utilization | TAVR Utilization | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EVAR | LVAD | TAVR | EVAR/TAVR Population | LVAD Population | EVAR | LVAD | TAVR | EVAR/TAVR Population | LVAD Population | Ontario | NY | P-value | Ontario | NY | P-value | Ontario | NY | P-value | |
| Total | 3322 | 139 | 1722 | 6917889 | 11001544 | 6360 | 696 | 4853 | 9156195 | 15053173 | 12.8 | 20.1 | <.001 | 0.3 | 1.3 | <.001 | 6.6 | 14.3 | <.001 |
| Age group | |||||||||||||||||||
| Age<60 | 94 | 87 | 23 | 3967710 | 8051365 | 337 | 343 | 49 | 5471992 | 11368970 | 0.6 | 1.7 | <.001 | 0.3 | 0.8 | <.001 | 0.2 | 0.2 | .094 |
| Age, 60–69, | 696 | 41 | 94 | 1501136 | 1501136 | 1513 | 242 | 267 | 1839471 | 1839471 | 12.4 | 22.6 | <.001 | 0.7 | 3.6 | <.001 | 1.7 | 3.9 | <.001 |
| Age, 70–79, | 1,431 | 11 | 417 | 877739 | 877739 | 2433 | 103 | 976 | 1062198 | 1062198 | 43.5 | 63.6 | <.001 | 0.3 | 2.7 | <.001 | 12.7 | 24.7 | <.001 |
| Age, 80+ | 1,101 | 0 | 1188 | 571304 | 571304 | 2077 | 8 | 3561 | 782534 | 782534 | 51.4 | 75 | <.001 | 0.0 | 0.3 | 0.024 | 55.5 | 123.6 | <.001 |
| Sex group | |||||||||||||||||||
| Men | 2797 | 109 | 935 | 3316230 | 5346403 | 4950 | 552 | 2340 | 4237376 | 7165866 | 22.5 | 33.7 | <.001 | 0.5 | 2.2 | <.001 | 7.5 | 15.5 | <.001 |
| Women | 525 | 30 | 787 | 3601659 | 5655141 | 1410 | 144 | 2513 | 4918819 | 7887307 | 3.9 | 7.8 | <.001 | 0.1 | 0.5 | <.001 | 5.8 | 13.3 | <.001 |
Counts are total number of procedures, allowing for individual patients to undergo multiple procedures
Total Utilization for NY are directly standardized to match the age and sex of the Ontario population.
Figure.
100,000 population, per-year) of EVAR, LVAD, and TAVR in Ontario and New York, stratified by sex
In our analyses evaluating standardized per-capita utilization by neighborhood income, we did not find consistent differences in utilization of EVAR or LVAD among residents in lower income (quintile 1) or higher income (quintile 5) neighborhoods in either New York or Ontario (Table 4). Alternatively, for TAVR we found slightly lower utilization in Ontario for low income neighborhoods (quintile 1 utilization 5.7 per-100,000 per-year) compared to higher income neighborhoods (6.8 per-100,000; P=.033) and much lower utilization in New York (9.0 vs 22.0; P<.001). While all procedures in Ontario were paid for by the provincial insurance plan (OHIP), results from New York show significant differences by procedure (Supplemental Table 4). Medicare was the payer for 80% of EVARs, 93% of TAVRs but only 45% of LVADs; alternatively private insurance was the payer for 15% of EVARs, 5% of TAVRs, but 37% of LVADs.
Table 4:
Evaluation of differences in age/sex standardized utilization by neighborhood income quintile (quintile 1, lowest income: quintile 5, highest income)
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
|---|---|---|---|---|---|
| EVAR | |||||
| Ontario | 13.3 | 13.6 | 12.5 | 12.3 | 11.8 |
| New York | 16.8 | 23.2 | 21.3 | 19.8 | 18.6 |
| LVAD | |||||
| Ontario | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 |
| New York | 1.5 | 1.2 | 1.3 | 1.4 | 1.3 |
| TAVR | |||||
| Ontario | 5.7 | 7.1 | 6.7 | 6.2 | 6.8 |
| New York | 9.0 | 9.4 | 12.2 | 13.5 | 22.0 |
Utilization = procedures per-100,000 per-year.
The unadjusted hospital LOS was 0.3 days shorter in New York as compared to Ontario for EVAR (P=.06), 15 days shorter for LVAD (P<.001) and 1.6 days shorter for TAVR (P<.001)(Table 5). Mortality, both within 7-days of admission and in-hospital was lower in New York for all 3 procedures, with particularly large differences for LVAD and TAVR. For example, in-hospital death within 7-days of LVAD implantation occurred in 1.7% of patients in New York and 9.4% of patients in Ontario (P<.001). Among patients with TAVR, overall in-hospital mortality was 2.7% in New York compared with 5.2% in Ontario (P<.001). Risk-standardized outcomes generally demonstrated shorter LOS and higher 90-day readmission rates in New York (Table 6). Mortality in New York for all 3 procedures was lower by a clinically significant (if not statistically significant in all cases) magnitude (Table 6) irrespective of definition (in-hospital or within 7-days of procedure).
Table 5:
Unadjusted outcomes for recipients of EVAR, LVAD, and TAVR in Ontario and New York
| EVAR | LVAD | TAVR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ontario (N=3322) |
NY (N=6360) |
P-value | Ontario (N=139) |
NY (N=696) |
P-value | Ontario (N=1722) |
NY (N=4853) |
P-value | |
| Length-of-stay, mean (SD) | 4.1 (9.3) | 3.8 (6.2) | 0.0587 | 57.5(50.1) | 41 (34.9) | <.001 | 11.1 (15.4) | 9.5 (9.1) | <.001 |
| Length-of-stay, median (interquartile range) | 2 (1,4) | 2 (1, 4) | NA | 45(29,75) | 33 (23, 48) | 7 (4, 12) | 7 (4, 12) | NA | |
| Discharge disposition | |||||||||
| Died in-hospital, within 7-days of admission, number, (%) | 51 (1.5) | 62 (1.0) | 0.03 | 7 (5.0) | SC* | <.001 | 32 (1.9) | 51 (1.1) | 0.012 |
| Died in-hospital within 7-days of procedure, number (%) | 52 (1.6) | 66 (1.0) | 0.01 | 13 (9.4) | 12 (1.7) | <.001 | 48 (2.8) | 67 (1.4) | <.001 |
| Died in-hospital, number (%) | 69 (2.1) | 108 (1.7) | 0.1641 | 31 (22.3) | 34 (4.9) | <.001 | 90 (5.2) | 129 (2.7) | <.001 |
| Home, number (%) | 3,032 (91.3) | 5678 (89.3) | 0.0019 | 97 (69.8) | 481 (69.1) | 0.870 | 1,334 (77.5) | 3281 (67.6) | <.001 |
| Transfer to another acute-care hospital, number (%) | 92 (2.8) | 24 (0.4) | <.001 | SC* | SC* | 0.477 | 112 (6.5) | 24 (0.5) | <.001 |
| Post-acute-care, (%) | 70 (2.1) | 545 (8.6) | <.001 | 6–10(4.3–7.2)† | 179 (25.7) | <.001 | 184(10.7) | 1418 (29.2) | <.001 |
| Readmission | |||||||||
| 30-day hospital readmission, number (%) | 293 (9.0) | 707 (11.3) | 0.001 | 27 (25.0) | 158 (23.9) | 0.804 | 251 (15.3) | 822 (17.4) | 0.051 |
| 90-day hospital readmission, number (%) | 504 (15.5) | 1252 (20.0) | <.001 | 53 (49.1) | 255 (38.5) | 0.037 | 434 (26.6) | 1385 (29.3) | 0.038 |
SC = small cells, cells <6 for Ontario and <10 for NY can not be identified due to privacy regulations
Cells given as a range to comply with ICES policy regulations
Table 6:
Risk standardized outcome (point estimates and 95% confidence intervals) in Ontario and New York adjusted for demographics, neighborhood income, hospital procedure volume, and hospital length of stay
| EVAR | LVAD | TAVR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ontario | NY | P-value | Ontario | NY | P-value | Ontario | NY | P-value | |
| Hospital LOS, days (95% Confidence Intervals)* | 4.4 (3.8–5.0) | 3.7 (3.4–4) | 0.055 | 70.9 (58.8–82.9) | 39.2 (32.8–45.6) | <.001 | 10.4 (8.8–11.9) | 8.7 (8.2–9.3) | 0.050 |
| Readmission within 90-days of discharge, % (95% Confidence Intervals) | 11.4 (9.2–14.1) | 16.4 (14.2–19.0) | 0.090 | 37.5 (16.1–65.2) | 67.8 (46.4–99.0) | 0.841 | 20.1 (15.9–25.1) | 25.4 (20.9–30.8) | 0.951 |
| Mortality† | |||||||||
| In-hospital mortality, % (95% Confidence Intervals) | 2.7 (1.7–4.2) | 0.7 (0.4–1.2) | <.001 | 37.4 (15.2–66.6) | 4.1 (1.6–10.6) | 0.001 | 5.3 (3.2–8.5) | 2.1 (1.3–3.5) | 0.009 |
| Mortality within 7-days of procedure, Model 1,% (95% Confidence Intervals) | 1.6 (1.2–2.0) | 1.0 (0.8–1.3) | 0.029 | 8.4(4.2–15.9) | 2.6 (1.4–5) | 0.015 | 2.9(2.1–3.9) | 1.4 (1–1.8) | 0.001 |
| Mortality within 7-days of procedure, Model 2,% (95% Confidence Intervals) | 1.6 (1.2–2.1) | 0.8 (0.6–1.2) | 0.007 | 7.6 (4.0–14.0) | 2.4 (1.1–4.9) | 0.020 | 2.6(1.8–3.7) | 1.5 (0.8–2.8) | 0.147 |
| Mortality within 7-days of procedure, Model 3,% (95% Confidence Intervals) | 1.5 (1.2–2.1) | 0.8 (0.6–1.2) | 0.009 | 6.8 (3.5–12.6) | 3.8 (1.8–7.8) | 0.245 | 2.6(1.7–3.9) | 1.5 (0.8–2.9) | 0.164 |
Expected LOS for reference case derived from (age= mean age ON, sex= male, procedure volume= mean procedure volume ON, income group = 1st or 2nd quintile) based on GEE fits for each region.
Probability for reference case for mortality derived from (age= mean age ON, sex= male, procedure volume= mean procedure volume ON, income group = 1st or 2nd quintile, LOS= 1st quartile per ON) based on GEE fits for each region. For mortality within 7-days of procedure Model 1 adjusted for hospital volume, Model 2 for comorbidities listed in Table 1, Model 3 for volume plus comorbidity with the reference cases the same as described above.
Discussion
In analysis of administrative data from the US and Canada, we found substantial differences in the use of 3 advanced cardiovascular therapies. We found differences in socio-demographic characteristics of patients receiving EVAR, LVAD and TAVR in New York and Ontario. We found similar utilization rates for EVAR and LVAD for New York and Ontario residents irrespective of neighborhood income strata; alternatively, we found a strong neighborhood income gradient for TAVR in New York (lower utilization in lower income neighborhoods) but less so in Ontario. We found that a larger percentage of acute care hospitals in New York offered these therapies, but that New York had more low-volume hospitals particularly for EVAR. Most importantly, utilization rates of EVAR, LVAD, and TAVR were approximately 50%, 300% and 100% higher in New York than Ontario, with a particularly large New York-Ontario gradient observed in the elderly. In aggregate, these findings demonstrate markedly different access to and utilization of three advanced cardiovascular therapies for patients residing in two geographically proximate jurisdictions with vastly different healthcare systems.
A number of our findings warrant discussion. First, it is important to think about the causes of the higher utilization of each procedure in New York compared to Ontario. While increased utilization in New York could conceivably be attributed to differences in cardiovascular risk factors, this seems implausible given prior research.19, 20 Rather, we suspect that the higher utilization in New York reflects subtle differences in preferences and values of Americans and Canadians towards health care and the manner in which these values influence health care delivery.31, 32 Differing public values within countries are likely to influence everything including technology adoption, payment policy, physician supply and mix (e.g., specialists versus general practitioners), and the public’s demand for healthcare services.33 Prior investigations have suggested that these differences translate into less spending in the US on the social safety net, but greater spending on acute care.34
In the case of the advanced cardiovascular therapies that are the focus of our study there are several mechanisms that could contribute to the higher utilization in New York and lower utilization in Ontario. At a regulatory level, he US Food and Drug Administration (FDA) is charged with determining if a therapy is “safe and effective” and then granting approval for use.35 Historically, once approval was granted, the Centers for Medicare and Medicaid Services (CMS) placed few restrains on utilization and private insurers typically followed suit.36, 37 More recently, however, the FDA has increased oversight and requirements for post-marked surveillance of approved therapies while CMS has stipulated conditions for payment (e.g., hospital volume thresholds, participation in clinical registries) in an effort to enhance ability to monitor quality and safety.38, 39 For example CMS recently issued a 150-page national coverage determination that stipulated no-fewer-than 6 requirements for starting and maintaining a TAVR program (e.g., valve surgery volume, percutaneous coronary intervention volume, TAVR registry participation).40
At a healthcare financing level, both hospitals and physicians in the US are typically reimbursed for each procedure performed, thereby incentivizing volume. Moreover, as academic health centers adopt new therapies residents and fellows gain experience, increasing capacity and diffusion of innovation across the country.41
The regulatory environment in Ontario, like the rest of Canada, is quite different. As in the US provincial payers are judicious when approving hospital programs for new therapies such as EVAR, LVAD or TAVR. However, Ontario hospitals are typically funded for a fixed volume of procedures. While Ontario physicians are reimbursed on a fee-for-service basis, Ontario hospitals typically do not receive additional money for volumes exceeding specified thresholds.42, 43 Therefore, Ontario hospitals have substantial incentive to restrain EVAR, LVAD, and TAVR volumes even if physicians would perform more. To focus on LVAD for example, Canadian and US guidelines are generally similar and evolve in a similar direction as new evidence becomes available.44, 45 However in the US CMS began paying for LVADs for destination therapy in 2004, while OHIP only granted such approval in 2017 in very limited numbers.46 Similarly Canadian and US guidelines for TAVR are generally similar.47, 48 We suspect that recent studies demonstrating the effectiveness of TAVR in low-to-moderate risk patients will translate into a more rapid change in practice in the US relative to Canada.49, 50
Second, it is important to talk about how our work adds to the existing literature. Despite numerous “meta” studies comparing spending and mortality across countries using aggregated data (e.g., the Global Burden of Diseases studies),51, 52 there are very few contemporary studies comparing utilization and outcomes for specific diseases and procedures across countries and many do not focus on cardiovascular conditions.53, 54 Many of the existing cardiovascular studies are older19, 20 and more recent studies either do not include the US18 or do not focus on the utilization of advanced therapeutics.55–57
There are few studies describing per-capita utilization of EVAR, LVAD, or TAVR within single jurisdictions and almost none evaluating between-country differences. Data from Ontario reported EVAR utilization of 33 procedures per-100,000 population (age ≥ 65 years) per-year in 2009,12 while data demonstrated EVAR utilization in the US and England of approximately 40 and 14 per-100,000 (age ≥ 60 years) population in 2012 (England limits the use of EVAR because of the high upfront cost).58 Studies have documented increases in the number of LVADs implanted in the US over time,13, 14 but have not examined utilization rates. A recent study reported TAVR utilization was approximately 9 procedures per-100,000 US adults (age ≥ 18),59 but we are unaware of any international comparisons. Our findings of higher utilization of all 3 procedures (EVAR, LVAD, and TAVR) in New York extends prior research and provides benchmarks that can be used by policy makers and researchers.
Third, it is important to consider the potential impact of differential utilization rates. We do not know what the correct utilization rates for EVAR, LVAD, and TAVR are. We also do not know if the higher utilization in the New York is entirely explained by underuse in Ontario or is entirely indicative of overuse in New York; the truth is likely somewhere in between with a component of overuse in New York and underuse in Ontario whereby some patients who would benefit from EVAR, LVAD, and TAVR are not receiving treatment.1–3
Fourth, our study should be considered in light of ongoing concerns over access to care for lower income Americans. American values with respect to healthcare seem to differ from their peers in other developed countries in published surveys.31, 60 These differences in values seem to manifest themselves in surveys demonstrating that Americans are less likely to view differences in access to health care as fundamentally unjust31, 61 and a less robust social safety net. For decades there has been an assumption that the patchwork nature of health insurance was a principal cause of the widespread health disparities observed in the US,62 but very few studies have directly evaluated differential access to care or outcomes for patients of higher-and-lower socioeconomic status in the US relative to other countries. Much of the best work has come from Gorey et al who has methodically examined cancer care received by low-income Canadians and Americans.21, 63 Our study provides reassuring data that utilization of EVAR and LVAD is similar among adults residing in lower income and higher income neighborhoods in both New York and Ontario, suggesting that lower income in New York may not be a tremendous barrier to receipt of these therapies. In contrast, utilization of TAVR was much lower for adults residing in lower income neighborhoods relative to higher income neighborhoods in New York (less so in Ontario).
Fifth, it is important to mention the higher mortality that we observed in Ontario. While most obvious for LVAD, lower utilization rates for all procedures in Ontario likely results in significant differences in the patients receiving procedures in Ontario and New York (a type of referral bias). Our reliance on administrative data means that we lack the nuanced clinical data required for adequate risk adjustment. The clinically implausible differences in prevalence of comorbid conditions (fewer comorbid conditions for patients in Ontario) mirrors results of prior studies using cross-border administrative data23, 54 and likely represents differences in coding practices rather than true differences in comorbidity. Given that the higher mortality in Ontario was observed in several adjusted models and persisted whether we looked at peri-procedural mortality (within 7-days) or in-hospital, further evaluation using clinical registries with far richer data will be crucial.
A number of other findings merit brief mention. We found a significantly higher percentage of EVAR and TAVR in New York performed on women compared to Ontario; the explanation for this difference is unclear. Our finding that higher utilization of EVAR and TAVR in New York were magnified in the elderly is important and may suggest a difference between countries in willingness to offer costly interventions to the aged. The significantly higher rates of comorbid conditions observed in the New York cohorts mirror findings in other studies using hospital discharge data and require comment.23, 54 While it is possible that EVAR, LVAD, and TAVR recipients in New York are healthier than their Ontario counterparts, we are doubtful. Rather, the higher burden of comorbid conditions in New York likely reflect the incentives placed upon US hospitals to maximize coding of comorbid conditions,64 a pressure that does not exist in Canada. The differences in coding of comorbid conditions have major implications for cross border comparative research using administrative data.
Our study has several limitations that warrant mention. First, our analysis is limited to administrative data from 1 Canadian province and 1 US State and should be generalized with care. Second, our study did not capture New York residents who may have received surgery outside of New York, thus artifactually reducing the New York utilization rate. Third, we lacked the clinical detail to understand indications for each procedure; without this level of detail we are unable to determine whether our findings indicate overuse in New York, underuse in Ontario or some combination. Fourth, we lacked the ability to adequately assess adjusted differences in mortality or evaluate mortality that occurred after hospital discharge or functional outcomes. Finally, we did not examine other complementary cardiovascular procedures including open aortic aneurysm repair and surgical aortic valve replacement that would influence the total number of aneurysm and aortic valve procedures performed in each jurisdiction. Future research should examine the balance between EVAR and open AAA repair, TAVR and surgical AVR as well as longer term trends in adoption and de-adoption of cardiovascular therapies.
In aggregate, our findings demonstrate marked variation in access to and utilization of EVAR, LVAD, and TAVR for populations residing in two geographically proximate jurisdictions with vastly different healthcare systems. Policymakers on both sides of the border would do well to contemplate how to reconcile finite budgets, potentially insatiable demand, and equitable access for effective but costly cardiovascular therapies.
Supplementary Material
What is known
High healthcare spending in the US relative to other developed countries is thought to be due, in part, to higher utilization of costly procedures.
There is little empirical data evaluating differences in utilization of advanced cardiovascular therapies (CV) in different countries.
What the study adds
While age of endovascular aortic aneurysm repair (EVAR), left ventricular assist device (LVAD), and transcatheter aortic valve replacement (TAVR) recipients was similar in New York and Ontario, recipients of EVAR and TAVR in Ontario were significantly less likely to be female relative to New York
We found that per-capita utilization of EVAR, LVAD, and TAVR were 57%, 430%, and 210% higher in New York State compared to Ontario.
Our study provides evidence of substantial between-country differences in how advanced CV therapies are used.
Sources of Funding:
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed in the material are those of the authors and not necessarily those of CIHI. Dr. Ko is supported by a Mid Career Award from the Heart and Stroke Foundation. Dr. Girotra is supported by a career development award (K08HL122527) from the National Heart Lung and Blood Institute and the Veterans Affairs Health Services Research & Development Award (I21HX002365). Dr. Lee is supported by a mid-career investigator from the Heart and Stroke Foundation and is the Ted Rogers Chair in Heart Function Outcomes. This work is also supported by a grant from the US National Institute of Aging to Drs. Cram and Landon (R01AG058878).
Footnotes
Author conflict of interest disclosures: None.
References
- 1.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH and Milano CA. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med. 2017;376:451–460. [DOI] [PubMed] [Google Scholar]
- 2.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW and Kappetein AP. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 3.Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–86. [DOI] [PubMed] [Google Scholar]
- 4.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J and Landon BE. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015;373:328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapadia SR, Tuzcu EM, Makkar RR, Svensson LG, Agarwal S, Kodali S, Fontana GP, Webb JG, Mack M, Thourani VH, Babaliaros VC, Herrmann HC, Szeto W, Pichard AD, Williams MR, Anderson WN, Akin JJ, Miller DC, Smith CR and Leon MB. Long-term outcomes of inoperable patients with aortic stenosis randomly assigned to transcatheter aortic valve replacement or standard therapy. Circulation. 2014;130:1483–92. [DOI] [PubMed] [Google Scholar]
- 6.Duncan A, Ludman P, Banya W, Cunningham D, Marlee D, Davies S, Mullen M, Kovac J, Spyt T and Moat N. Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: the U.K. Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv. 2015;8:645–53. [DOI] [PubMed] [Google Scholar]
- 7.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML and Starnes BW. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e2. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd and Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 9.Lamph S. Regulation of medical devices outside the European Union. Journal of the Royal Society of Medicine. 2012;105 Suppl 1:S12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squires DA. Explaining high health care spending in the United States: an international comparison of supply, utilization, prices, and quality. Issue Brief (Commonw Fund). 2012;10:1–14. [PubMed] [Google Scholar]
- 11.Papanicolas I and Jha AK. Challenges in International Comparison of Health Care Systems. Jama. 2017;318:515–516. [DOI] [PubMed] [Google Scholar]
- 12.Jetty P and Husereau D. Trends in the utilization of endovascular therapy for elective and ruptured abdominal aortic aneurysm procedures in Canada. J Vasc Surg. 2012;56:1518–26, 1526.e1. [DOI] [PubMed] [Google Scholar]
- 13.Briasoulis A, Inampudi C, Akintoye E, Adegbala O, Asleh R, Alvarez P and Bhama J. Regional Variation in Mortality, Major Complications, and Cost After Left Ventricular Assist Device Implantation in the United States (2009 to 2014). Am J Cardiol. 2018;121:1575–1580. [DOI] [PubMed] [Google Scholar]
- 14.Shah N, Agarwal V, Patel N, Deshmukh A, Chothani A, Garg J, Badheka A, Martinez M, Islam N and Freudenberger R. National Trends in Utilization, Mortality, Complications, and Cost of Care After Left Ventricular Assist Device Implantation From 2005 to 2011. Ann Thorac Surg. 2016;101:1477–84. [DOI] [PubMed] [Google Scholar]
- 15.Hannan EL, Samadashvili Z, Stamato NJ, Lahey SJ, Wechsler A, Jordan D, Sundt TM 3rd, Gold JP, Ruiz CE, Ashraf MHand Smith CR. Utilization and 1-Year Mortality for Transcatheter Aortic Valve Replacement and Surgical Aortic Valve Replacement in New York Patients With Aortic Stenosis: 2011 to 2012. JACC Cardiovasc Interv. 2016;9:578–85. [DOI] [PubMed] [Google Scholar]
- 16.Mylotte D, Osnabrugge RLJ, Windecker S, Lefevre T, de Jaegere P, Jeger R, Wenaweser P, Maisano F, Moat N, Sondergaard L, Bosmans J, Teles RC, Martucci G, Manoharan G, Garcia E, Van Mieghem NM, Kappetein AP, Serruys PW, Lange R and Piazza N. Transcatheter aortic valve replacement in Europe: adoption trends and factors influencing device utilization. J Am Coll Cardiol. 2013;62:210–219. [DOI] [PubMed] [Google Scholar]
- 17.Baicker K and Chandra A. Challenges in Understanding Differences in Health Care Spending Between the United States and Other High-Income Countries. Jama. 2018;319:986–987. [DOI] [PubMed] [Google Scholar]
- 18.Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, Jernberg T and Hemingway H. Acute myocardial infarction: a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet. 2014;383:1305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko DT, Krumholz HM, Wang Y, Foody JM, Masoudi FA, Havranek EP, You JJ, Alter DA, Stukel TA, Newman AM and Tu JV. Regional differences in process of care and outcomes for older acute myocardial infarction patients in the United States and Ontario, Canada. Circulation. 2007;115:196–203. [DOI] [PubMed] [Google Scholar]
- 20.Ko DT, Tu JV, Masoudi FA, Wang Y, Havranek EP, Rathore SS, Newman AM, Donovan LR, Lee DS, Foody JM and Krumholz HM. Quality of care and outcomes of older patients with heart failure hospitalized in the United States and Canada. Arch Intern Med. 2005;165:2486–92. [DOI] [PubMed] [Google Scholar]
- 21.Gorey KM, Fung KY, Luginaah IN, Holowaty EJ and Hamm C. Income and long-term breast cancer survival: comparisons of vulnerable urban places in Ontario and California. The breast journal. 2010;16:416–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorey KM, Luginaah IN, Bartfay E, Fung KY, Holowaty EJ, Wright FC, Hamm C and Kanjeekal SM. Effects of socioeconomic status on colon cancer treatment accessibility and survival in Toronto, Ontario, and San Francisco, California, 1996–2006. Am J Public Health. 2011;101:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cram P, Landon BE, Matelski J, Ling V, Stukel TA, Paterson JM, Gandhi R, Hawker GA and Ravi B. Utilization and Short-Term Outcomes of Primary Total Hip and Knee Arthroplasty in the United States and Canada: An Analysis of New York and Ontario Administrative Data. Arthritis & rheumatology (Hoboken, NJ). 2018;70:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE and Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 25.White C. Cutting medicare hospital prices leads to a spillover reduction in hospital discharges for the nonelderly. Health Serv Res. 2014;49:1578–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elixhauser A, Steiner C, Harris DR and Coffey RM. Comorbidity measures for use with administrative data. Medical Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 27.Kolte D, Khera S, Sardar MR, Gheewala N, Gupta T, Chatterjee S, Goldsweig A, Aronow WS, Fonarow GC, Bhatt DL, Greenbaum AB, Gordon PC, Sharaf B and Abbott JD. Thirty-Day Readmissions After Transcatheter Aortic Valve Replacement in the United States: Insights From the Nationwide Readmissions Database. Circulation Cardiovascular interventions. 2017;10. [DOI] [PubMed] [Google Scholar]
- 28.Khazanie P, Hammill BG, Patel CB, Eapen ZJ, Peterson ED, Rogers JG, Milano CA, Curtis LH and Hernandez AF. Trends in the use and outcomes of ventricular assist devices among medicare beneficiaries, 2006 through 2011. J Am Coll Cardiol. 2014;63:1395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jetty P, Hebert P and van Walraven C. Long-term outcomes and resource utilization of endovascular versus open repair of abdominal aortic aneurysms in Ontario. J Vasc Surg. 2010;51:577–83, 583.e1–3. [DOI] [PubMed] [Google Scholar]
- 30.Schermerhorn ML, Bensley RP, Giles KA, Hurks R, O’Malley AJ, Cotterill P, Chaikof E and Landon BE. Changes in abdominal aortic aneurysm rupture and short-term mortality, 1995–2008: a retrospective observational study. Ann Surg. 2012;256:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von dem Knesebeck O, Vonneilich N and Kim TJ. Are health care inequalities unfair? A study on public attitudes in 23 countries. International journal for equity in health. 2016;15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivers N, Brown AD and Detsky AS. Lessons From the Canadian Experience With Single-Payer Health Insurance: Just Comfortable Enough With the Status Quo. JAMA Intern Med. 2018;178:1250–1255. [DOI] [PubMed] [Google Scholar]
- 33.van Exel J, Baker R, Mason H, Donaldson C and Brouwer W. Public views on principles for health care priority setting: findings of a European cross-country study using Q methodology. Soc Sci Med. 2015;126:128–37. [DOI] [PubMed] [Google Scholar]
- 34.Bradley EH, Canavan M, Rogan E, Talbert-Slagle K, Ndumele C, Taylor L and Curry LA. Variation In Health Outcomes: The Role Of Spending On Social Services, Public Health, And Health Care, 2000–09. Health Aff (Millwood). 2016;35:760–8. [DOI] [PubMed] [Google Scholar]
- 35.Van Norman GA. Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices. JACC Basic to translational science. 2016;1:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chambers JD, Morris S, Neumann PJ and Buxton MJ. Factors predicting Medicare national coverage: an empirical analysis. Med Care. 2012;50:249–56. [DOI] [PubMed] [Google Scholar]
- 37.Tunis SR. Why Medicare has not established criteria for coverage decisions. N Engl J Med. 2004;350:2196–8. [DOI] [PubMed] [Google Scholar]
- 38.Pease AM, Krumholz HM, Downing NS, Aminawung JA, Shah ND and Ross JS. Postapproval studies of drugs initially approved by the FDA on the basis of limited evidence: systematic review. BMJ. 2017;357:j1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roginiel AC, Dhruva SS and Ross JS. Evidence supporting FDA approval and CMS national coverage determinations for novel medical products, 2005 through 2016: A cross-sectional study. Medicine. 2018;97:e12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Medicare and Medicaid Services. Decision Memo for Transcatheter Aortic Valve Replacement (TAVR). 2019.
- 41.Groeneveld PW, Epstein AJ, Yang F, Yang L and Polsky D. Medicare’s policy on carotid stents limited use to hospitals meeting quality guidelines yet did not hurt disadvantaged. Health Aff (Millwood). 2011;30:312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detsky AS, Abrams HB, Ladha L and Stacey SR. Global budgeting and the teaching hospital in Ontario. Med Care. 1986;24:89–94. [DOI] [PubMed] [Google Scholar]
- 43.Palmer KS, Brown AD, Evans JM, Marani H, Russell KK, Martin D and Ivers NM. Standardising costs or standardising care? Qualitative evaluation of the implementation and impact of a hospital funding reform in Ontario, Canada. Health research policy and systems. 2018;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKelvie RS, Moe GW, Cheung A, Costigan J, Ducharme A, Estrella-Holder E, Ezekowitz JA, Floras J, Giannetti N, Grzeslo A, Harkness K, Heckman GA, Howlett JG, Kouz S, Leblanc K, Mann E, O’Meara E, Rajda M, Rao V, Simon J, Swiggum E, Zieroth S, Arnold JM, Ashton T, D’Astous M, Dorian P, Haddad H, Isaac DL, Leblanc MH, Liu P, Sussex B and Ross HJ. The 2011 Canadian Cardiovascular Society heart failure management guidelines update: focus on sleep apnea, renal dysfunction, mechanical circulatory support, and palliative care. Can J Cardiol. 2011;27:319–38. [DOI] [PubMed] [Google Scholar]
- 45.Cook JL, Colvin M, Francis GS, Grady KL, Hoffman TM, Jessup M, John R, Kiernan MS, Mitchell JE, Pagani FD, Petty M, Ravichandran P, Rogers JG, Semigran MJ and Toole JM. Recommendations for the Use of Mechanical Circulatory Support: Ambulatory and Community Patient Care: A Scientific Statement From the American Heart Association. Circulation. 2017;135:e1145–e1158. [DOI] [PubMed] [Google Scholar]
- 46.Left Ventricular Assist Devices for Destination Therapy: A Health Technology Assessment. Ontario health technology assessment series. 2016;16:1–60. Available at: http://www.hqontario.ca/Portals/0/Documents/evidence/reports/hta-lvad-1602-en.pdf [Accessed December 4, 2019]. [PMC free article] [PubMed] [Google Scholar]
- 47.Webb J, Rodes-Cabau J, Fremes S, Pibarot P, Ruel M, Ibrahim R, Welsh R, Feindel C and Lichtenstein S. Transcatheter aortic valve implantation: a Canadian Cardiovascular Society position statement. Can J Cardiol. 2012;28:520–8. [DOI] [PubMed] [Google Scholar]
- 48.Otto CM, Kumbhani DJ, Alexander KP, Calhoon JH, Desai MY, Kaul S, Lee JC, Ruiz CE and Vassileva CM. 2017 ACC Expert Consensus Decision Pathway for Transcatheter Aortic Valve Replacement in the Management of Adults With Aortic Stenosis: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2017;69:1313–1346. [DOI] [PubMed] [Google Scholar]
- 49.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest, Tchetche D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh J, Boulware MJ, Qiao H, Mugglin AS and Reardon MJ. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 50.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe JM, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG and Smith CR. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 51.Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391:2236–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papanicolas I, Woskie LR and Jha AK. Health Care Spending in the United States and Other High-Income Countries. Jama. 2018;319:1024–1039. [DOI] [PubMed] [Google Scholar]
- 53.Cram P, Lix LM, Bohm E, Yan L, Roos L, Matelski J, Gandhi R, Landon B and Leslie WD. Hip fracture care in Manitoba, Canada and New York State, United States: an analysis of administrative data. CMAJ open. 2019;7:E55–E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cram P, Landon BE, Matelski J, Ling V, Perruccio AV, Paterson JM and Rampersaud YR. Utilization and Outcomes for Spine Surgery in the United States and Canada. Spine (Phila Pa 1976). 2019;44:1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapsomaniki E, Thuresson M, Yang E, Blin P, Hunt P, Chung S-C, Stogiannis D, Pujades-Rodriguez M, Timmis A, Denaxas SC, Danchin N, Stokes M, Thomas-Delecourt F, Emmas C, Hasvold P, Jennings E, Johansson S, Cohen DJ, Jernberg T, Moore N, Janzon M and Hemingway H. Using big data from health records from four countries to evaluate chronic disease outcomes: a study in 114 364 survivors of myocardial infarction. European Heart Journal - Quality of Care and Clinical Outcomes. 2016;2:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNamara RL, Chung SC, Jernberg T, Holmes D, Roe M, Timmis A, James S, Deanfield J, Fonarow GC, Peterson ED, Jeppsson A and Hemingway H. International comparisons of the management of patients with non-ST segment elevation acute myocardial infarction in the United Kingdom, Sweden, and the United States: The MINAP/NICOR, SWEDEHEART/RIKS-HIA, and ACTION Registry-GWTG/NCDR registries. International journal of cardiology. 2014;175:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samsky MD, Ambrosy AP, Youngson E, Liang L, Kaul P, Hernandez AF, Peterson ED and McAlister FA. Trends in Readmissions and Length of Stay for Patients Hospitalized With Heart Failure in Canada and the United States. JAMA cardiology. 2019;4:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karthikesalingam A, Vidal-Diez A, Holt PJ, Loftus IM, Schermerhorn ML, Soden PA, Landon BE and Thompson MM. Thresholds for Abdominal Aortic Aneurysm Repair in England and the United States. N Engl J Med. 2016;375:2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta T, Kalra A, Kolte D, Khera S, Villablanca PA, Goel K, Bortnick AE, Aronow WS, Panza JA, Kleiman NS, Abbott JD, Slovut DP, Taub CC, Fonarow GC, Reardon MJ, Rihal CS, Garcia MJ and Bhatt DL. Regional Variation in Utilization, In-hospital Mortality, and Health-Care Resource Use of Transcatheter Aortic Valve Implantation in the United States. Am J Cardiol. 2017;120:1869–1876. [DOI] [PubMed] [Google Scholar]
- 60.Bradley EH, Sipsma H and Taylor LA. American health care paradox-high spending on health care and poor health. QJM : monthly journal of the Association of Physicians. 2017;110:61–65. [DOI] [PubMed] [Google Scholar]
- 61.Hero JO, Zaslavsky AM and Blendon RJ. The United States Leads Other Nations In Differences By Income In Perceptions Of Health And Health Care. Health Aff (Millwood). 2017;36:1032–1040. [DOI] [PubMed] [Google Scholar]
- 62.Woolhandler S and Himmelstein DU. A national health program: northern light at the end of the tunnel. Jama. 1989;262:2136–7. [PubMed] [Google Scholar]
- 63.Gorey KM, Luginaah IN, Bartfay E, Zou G, Haji-Jama S, Holowaty EJ, Hamm C, Kanjeekal SM, Wright FC, Balagurusamy MK and Richter NL. Better colon cancer care for extremely poor Canadian women compared with American women. Health & social work. 2013;38:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaughan-Sarrazin MS, Lu X and Cram P. The impact of paradoxical comorbidities on risk-adjusted mortality of Medicare beneficiaries with cardiovascular disease. Medicare Medicaid Res Rev. 2011;1:E1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.