Figure 2.
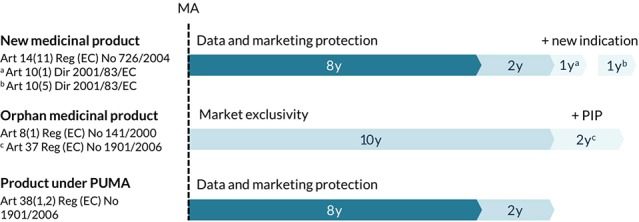
Overview of regulatory exclusivities relevant to the development of new uses in Europe. Marketing authorization (MA), pediatric investigation plan (PIP), pediatric-use marketing authorization (PUMA), regulation (Reg), directive (Dir).

Overview of regulatory exclusivities relevant to the development of new uses in Europe. Marketing authorization (MA), pediatric investigation plan (PIP), pediatric-use marketing authorization (PUMA), regulation (Reg), directive (Dir).