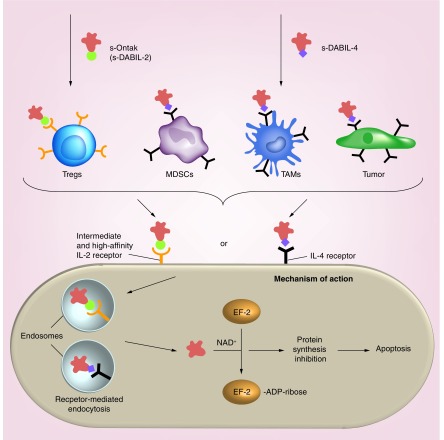
Figure 4. . Cartoon depictions of the mechanism of action of s-DAB389IL-2 and s-DAB389IL-4 which are produced as secreted proteins using Corynebacterium diphtheriae and are soluble, fully monomeric versions of DAB389IL-2 and DAB389IL-4.
Cartoon depiction of Treg-targeting action of s-DABIL-2 (targeting the IL-2 receptor, CD25) and the MDSC-, TAM-, and tumor-targeting action of s-DABIL-4 (targeting the IL-4 receptor, CD124 [Parveen et al., unpublished data]). Both targeted toxins bind to their cognate receptor, undergo receptor-mediated endocytosis with vesicular acidification and subsequently deliver their catalytic domains (red) to the cytosol of the targeted cell. The catalytic domain, a potent ADP-ribosyltransferase of elongation factor-2 (EF-2), enzymatically shuts down protein synthesis leading to apoptotic cell death.