Abstract
Background
Hip fracture is a major injury that causes significant problems for affected individuals and their family and carers. Over 40% of people with hip fracture have dementia or cognitive impairment. The outcomes of these individuals after surgery are poorer than for those without dementia. It is unclear which care and rehabilitation interventions achieve the best outcomes for these people. This is an update of a Cochrane Review first published in 2013.
Objectives
(a) To assess the effectiveness of models of care including enhanced rehabilitation strategies designed specifically for people with dementia following hip fracture surgery compared to usual care.
(b) To assess for people with dementia the effectiveness of models of care including enhanced rehabilitation strategies that are designed for all older people, regardless of cognitive status, following hip fracture surgery, compared to usual care.
Search methods
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group Specialised Register, MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), CINAHL (EBSCOhost), Web of Science Core Collection (ISI Web of Science), LILACS (BIREME), ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform on 16 October 2019.
Selection criteria
We included randomised and quasi‐randomised controlled trials evaluating the effectiveness of any model of enhanced care and rehabilitation for people with dementia after hip fracture surgery compared to usual care.
Data collection and analysis
Two review authors independently selected trials for inclusion and extracted data. We assessed risk of bias of the included trials. We synthesised data only if we considered the trials to be sufficiently homogeneous in terms of participants, interventions, and outcomes. We used the GRADE approach to rate the overall certainty of evidence for each outcome.
Main results
We included seven trials with a total of 555 participants. Three trials compared models of enhanced care in the inpatient setting with conventional care. Two trials compared an enhanced care model provided in inpatient settings and at home after discharge with conventional care. Two trials compared geriatrician‐led care in‐hospital to conventional care led by the orthopaedic team. None of the interventions were designed specifically for people with dementia, therefore the data included in the review were from subgroups of people with dementia or cognitive impairment participating in randomised controlled trials investigating models of care for all older people following hip fracture. The end of follow‐up in the trials ranged from the point of acute hospital discharge to 24 months after discharge.
We considered all trials to be at high risk of bias in more than one domain. As subgroups of larger trials, the analyses lacked power to detect differences between the intervention groups. Furthermore, there were some important differences in baseline characteristics of participants between the experimental and control groups. Using the GRADE approach, we downgraded the certainty of the evidence for all outcomes to low or very low.
The effect estimates for almost all comparisons were very imprecise, and the overall certainty for most results was very low. There were no data from any study for our primary outcome of health‐related quality of life. There was only very low certainty for our other primary outcome, activities of daily living and functional performance, therefore we were unable to draw any conclusions with confidence. There was low‐certainty that enhanced care and rehabilitation in‐hospital may reduce rates of postoperative delirium (odds ratio 0.04, 95% confidence interval (CI) 0.01 to 0.22, 2 trials, n = 141) and very low‐certainty associating it with lower rates of some other complications. There was also low‐certainty that, compared to orthopaedic‐led management, geriatrician‐led management may lead to shorter hospital stays (mean difference 4.00 days, 95% CI 3.61 to 4.39, 1 trial, n = 162).
Authors' conclusions
We found limited evidence that some of the models of enhanced rehabilitation and care used in the included trials may show benefits over usual care for preventing delirium and reducing length of stay for people with dementia who have been treated for hip fracture. However, the certainty of these results is low. Data were available from only a small number of trials, and the certainty for all other results is very low. Determining the optimal strategies to improve outcomes for this growing population of patients should be a research priority.
Keywords: Aged; Aged, 80 and over; Humans; Patient Care Team; Activities of Daily Living; Delirium; Delirium/prevention & control; Dementia; Dementia/complications; Hip Fractures; Hip Fractures/rehabilitation; Hip Fractures/surgery; Quality of Life; Randomized Controlled Trials as Topic
Plain language summary
Rehabilitation for people with dementia following a hip fracture operation
Background
Hip fracture is an injury primarily of elderly people, which is usually caused by a fall. It can affect a person's ability to walk, perform activities of daily living, and remain independent. Hip fracture is more common in people with dementia, and these individuals can find it more difficult to recover because they are at greater risk of becoming more confused and developing additional complications such as pressure sores and chest infections after surgery. They may also find it more difficult to express pain and discomfort.
Review question
We wanted to learn whether different ways of treating people with dementia following hip fracture might affect how well they recover and what the associated costs of their recovery might be. This is an update of a previous Cochrane Review.
Study characteristics
We searched for randomised controlled trials (a type of study in which participants are assigned to one of two or more treatment groups using a random method) that compared any model of enhanced care and rehabilitation for people with dementia after hip fracture versus the usual care provided in the trial setting. The latest search was performed on 16 October 2019.
We identified seven trials that studied a total of 555 people with dementia following hip fracture. Five trials compared an enhanced interdisciplinary rehabilitation and care programme where the various healthcare professionals worked collaboratively across hospital and community settings or just in hospital, to usual hospital care. Two trials compared care in‐hospital led by a geriatrician versus care led by an orthopaedic surgeon.
Key findings
People with dementia who receive enhanced care and rehabilitation in hospital after a hip fracture may be less likely to develop delirium. When care is led by a geriatrician, they may have stays in hospital that are three to four days shorter than if care is led by an orthopaedic surgeon. There was no information on the effect of any of the care models on quality of life, and we could not be certain about their effects on other important outcomes such as an individual's ability to manage their daily activities, regaining mobility, cognitive function, pain, death rates, or the likelihood of the person returning to the same place they had been living before the fracture.
Quality of the evidence
The main issues with the evidence were that most of the studies were small and their results may have been subject to bias. Most of the results of the review are very uncertain. None of the care models had been designed specifically for people with dementia. All of the data included in the review came from people with dementia who had been included in larger trials for all older people with hip fractures, although people with dementia may have particular needs.
Conclusions
There may be some benefits from the care models studied, but the currently available research is insufficient to determine the best ways to care for people with dementia after a hip fracture operation.
Summary of findings
Summary of findings for the main comparison. Interdisciplinary enhanced rehabilitation (inpatient rehabilitation) compared to conventional rehabilitation for adults with dementia following hip fracture surgery.
| Interdisciplinary geriatric rehabilitation (inpatient rehabilitation) compared to conventional rehabilitation for adults with dementia following hip fracture surgery | ||||||
| Patient or population: adults with dementia following hip fracture surgery Setting: hospital ward (inpatient) Intervention: interdisciplinary geriatric rehabilitation (inpatient rehabilitation) Comparison: conventional rehabilitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with conventional rehabilitation | Risk with interdisciplinary enhanced rehabilitation (inpatient rehabilitation) | |||||
| Health‐related quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Activities of daily living and functional performance (personal ADL independence at 12 months follow‐up) | 0/28 (0%) participants returned to functional independence at 12 months. | 1/19 (5%) participant returned to independence at 12 months. | OR 4.62 (0.18 to 119.63) | 47 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 |
The evidence for this result is very uncertain. |
| Activities of daily living and functional performance (walking independently without an aid or assistance at 12‐month follow‐up) | 1/28 (4%) participant returned to walking independence at 12 months. | 4/19 (21%) participants returned to walking independence at 12 months. | OR 7.20 (0.74 to 70.42) | 47 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 |
The evidence for this result is very uncertain. |
| Cognitive function | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Behaviour | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Pain | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Mortality (during hospitalisation) | Study population | OR 0.60 (0.17 to 2.13) | 152 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 | The evidence for this result is very uncertain. | |
| 125 participants per 1000 | 79 participants per 1000 (20 to 176) | |||||
| Adverse events (postoperative delirium during hospitalisation) | Study population | OR 0.04 (0.01 to 0.22) | 141 (2 RCTs) | ⊕⊝⊝⊝ LOW3 | The enhanced rehabilitation intervention may reduce the incidence of delirium. | |
| 662 participants per 1000 | 73 participants per 1000 (19 to 301) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADLs: activities of daily living; CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by three levels from high‐ to very low‐certainty in the outcome due to serious concern about risk of bias (performance, detection, contamination, and attrition bias with a risk of baseline imbalance) and very serious concern about imprecision due to small sample size, data from a single trial, and wide confidence interval (Stenvall 2012). 2Downgraded by three levels from high‐ to very low‐certainty in the outcome due to serious concern about risk of bias (performance and contamination bias (Freter 2017; Stenvall 2012; Uy 2008), detection bias (Freter 2017; Stenvall 2012), attrition bias (Stenvall 2012), selection bias (Freter 2017), and a risk of baseline imbalance (Freter 2017; Stenvall 2012)) and very serious concern about imprecision due to small sample size and wide confidence interval. 3Downgraded by two levels from high‐ to low‐certainty in the outcome due to serious concern about risk of bias (performance, detection, and contamination bias (Freter 2017; Stenvall 2012), attrition bias (Stenvall 2012), selection bias (Freter 2017), and a risk of baseline imbalance (Freter 2017; Stenvall 2012)) and serious concern about imprecision due to small sample sizes.
Summary of findings 2. Interdisciplinary enhanced rehabilitation (inpatient and community rehabilitation) compared to conventional rehabilitation for adults with dementia following hip fracture surgery.
| Interdisciplinary geriatric rehabilitation (inpatient and community rehabilitation) compared to conventional rehabilitation for adults with dementia following hip fracture surgery | ||||||
| Patient or population: adults with dementia following hip fracture surgery Setting: hospital ward (inpatient) and community (home‐based) Intervention: interdisciplinary geriatric rehabilitation (inpatient and community rehabilitation) Comparison: conventional rehabilitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with conventional rehabilitation | Risk with interdisciplinary enhanced rehabilitation (inpatient and community rehabilitation) | |||||
| Health‐related quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Activities of daily living and functional performance (Chinese Barthel Index (0‐to‐100‐point scale where higher scores indicate greater functional performance at 12 months) |
The mean function in the control group was 68.4 points. | The mean function was 25.4 points higher (10.9 to 39.9 points higher). | ‐ | 36 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | The evidence for this result is very uncertain. |
| Activities of daily living and functional performance (pre‐fracture walking levels at 12 months) | 7/19 (37%) participants regained pre‐fracture walking levels. | 17/17 (100%) participants regained pre‐fracture walking levels. | OR 58.33 (3.04 to 1118.19) | 36 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | The evidence for this result is very uncertain. |
| Cognitive function | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Behaviour | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Pain | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Mortality at 12 months post‐hip fracture | Study population | OR 1.07 (0.47 to 2.45) | 177 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 | The evidence for this result is very uncertain. | |
| 146 participants per 1000 | 155 participants per 1000 (75 to 296) | |||||
| Adverse events (incidence of falls at 12 months) | 2/19 (11%) participants experienced a fall. | 0/17 (0%) reported. | OR 0.20 (0.01 to 4.47) | 36 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | The evidence for this result is very uncertain. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by three levels from high‐ to very low‐certainty in the outcome due to serious concern about risk of bias (performance, detection, and contamination bias) and very serious concern about imprecision due to small sample size, data from a single trial, and wide confidence interval (Shyu 2012). 2Downgraded by three levels from high‐ to very low‐certainty in the outcome due to serious concern about risk of bias (selection, attrition, and reporting bias for both trials (Huusko 2000; Shyu 2012), contamination bias (Shyu 2012), and a risk of baseline imbalance (Huusko 2000)) and very serious concern about imprecision due to small sample size and wide confidence interval.
Summary of findings 3. Geriatrician‐led inpatient management compared to orthopaedic‐led inpatient management for adults with dementia following hip fracture surgery.
| Geriatrician‐led inpatient management compared to orthopaedic‐led inpatient management for adults with dementia following hip fracture surgery | ||||||
| Patient or population: adults with dementia following hip fracture surgery Setting: hospital ward (inpatient) Intervention: geriatrician‐led inpatient management Comparison: orthopaedic‐led inpatient management | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with orthopaedic‐led inpatient management | Risk with geriatrician‐led inpatient management | |||||
| Health‐related quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Activities of daily living and functional performance (activities of daily living capabilities assessed using BADLS at 12 months) BADLS (0 to 60 points; higher scores equate to poorer functional performance) |
The mean BADLS function in the control group was 11.0 points. | The mean BADLS function was 1.5 points lower (3.92 lower to 0.92 points higher). | ‐ | 87 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | The evidence for this result is very uncertain. |
| Activities of daily living and functional performance (NEADL score at 12 months) NEADL (0 to 22 points, where higher scores equate to greater functional performance) |
The mean NEADL function in the control group was 13.6 points. | The mean NEADL function was 3 points lower (8.11 lower to 2.11 points higher). | ‐ | 87 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | The evidence for this result is very uncertain. |
| Activities of daily living and functional performance (functional performance measured using the SPPB at 12 months) SPPB (0 to 12 points, where higher scores equate to greater functional performance) |
The mean SPPB function in the control group was 1.9 points. | The mean SPPB function was 0.3 point higher (0.65 lower to 1.25 points higher). | ‐ | 87 (1 RCT) | ⊕⊝⊝⊝ VERY LOW2 | The evidence for this result is very uncertain. |
| Cognitive function (cognitive function measured using the IQCODE at 12 months) IQCODE (1 to 5 points, where higher scores equate to poorer cognitive function) | The mean IQCODE score in the control group was 4.7 points. | The mean cognitive score was 0.1 points higher (0.18 lower to 0.38 higher). | ‐ | 87 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | The evidence for this result is very uncertain. |
| Behaviour | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Pain | ‐ | ‐ | ‐ | ‐ | ‐ | No data available on this outcome. |
| Mortality at 12 months | 33/41 (80%) participants died. | 31/46 (67%) participants died. | OR 2.00 (0.74 to 5.36) | 87 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | The evidence for this result is very uncertain. |
| Adverse events (delirium during hospitalisation) | Study population | OR 0.94 (0.52 to 1.72) | 212 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW3 | The evidence for this result is very uncertain. | |
| 721 participants per 1000 | 708 participants per 1000 (573 to 816) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BADLS: basic activities of daily living; CI: confidence interval; IQCODE: Informant Questionnaire on Cognitive Decline in the Elderly; NEADL: Nottingham Extended Activities of Daily Living Scale; OR: odds ratio; RCT: randomised controlled trial; SPPB: Short Performance Physical Battery | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by three levels from high‐ to very low‐certainty in the outcome due to serious concern about risk of bias (performance, attrition, and contamination bias) and very serious concern about imprecision due to small sample size, data from a single trial, and wide confidence interval (Wyller 2012). 2Downgraded by three levels from high‐ to very low‐certainty in the outcome due to serious concern about risk of bias (performance, attrition, and contamination bias) and very serious concern about imprecision due to small sample size, with the data originating from a single trial (Wyller 2012). 3Downgraded by three levels from high‐ to very low‐certainty in the outcome due to serious concern about risk of bias (performance and contamination bias (Marcantonio 2001; Wyller 2012), attrition bias (Wyller 2012), and a risk of baseline imbalance (Marcantonio 2001)) and very serious concern about imprecision due to small sample size, data from a single trial, and wide confidence interval (Wyller 2012).
Background
Description of the condition
The hip joint is the articulation between the thigh bone (femur) and the pelvis. The term ‘hip fracture’ encompasses all fractures of the upper (proximal) part of the thigh bone (femur). Hip fractures are commonly divided into two types: intracapsular fractures, which represent those that occur within or proximal to the attachment of the hip joint capsule to the femur, and extracapsular, which represent fractures occurring below or distal to the attachment of the hip joint capsule (Parker 2010). Hip fracture is a common injury in elderly people.
The majority of people undergo hip surgery following hip fracture (Uzoigwe 2012). The location of the fracture, stability, and degree of comminution (number of pieces the bone breaks into) determine which operative procedure should be used to repair the hip fracture. The aim of surgery, irrespective of the type of operation, is to reduce pain, facilitate early weight‐bearing mobility to improve outcome, and facilitate independence in activities of daily living, such as bathing, dressing, and continence (Handoll 2009). A delay in surgical intervention is known to be a key factor in producing poorer outcomes (Mattisson 2018).
The annual incidence rate of hip fracture has been estimated as 1.29/1000 person‐years in men and 2.24/1000 person‐years in women (Adams 2013). This figure is likely to rise over the next few years as the general population increases in age (Lewiecki 2018). It is the most common condition requiring physical rehabilitation in older adults (Lenze 2007), amongst both those who are cognitively healthy and those with all degrees of cognitive impairment (Morrison 2000). Hip fracture is associated with significant pain and loss of independence and function (Morrison 2000). Thirty‐three per cent to 37% of patients return to their prior level of function within six months, including those needing assistance (Tang 2017). However, only 24% of people are independently mobile six months after hip fracture (Magaziner 2002).
Dementia is a global loss of cognitive and intellectual functioning that gradually interferes with social and functional performance (Lieberman 2006; McGilton 2012). It is a common condition with a significant impact on society. It is expected that the number of people living with dementia will double worldwide to 75 million by 2030 and 131.5 million in 2050 (Alzheimer's Disease International 2020). A systematic review of observational studies found that 19% of people with hip fracture meet formal diagnostic criteria for dementia, and 42% are cognitively impaired (Seitz 2011b). It is expected that the number of people with dementia and hip fracture will increase during the next 25 years (Adunsky 2003b; Knapp 2007). Compared to those without dementia, community‐dwelling people with dementia have higher mortality after hip fracture and are more likely to be admitted to long‐term care (Seitz 2014). Health and social care expenditure in England on people with dementia in the year following admission for fractured neck of femur has been estimated to be in excess of GBP 1 billion (GBP 1037 million in 2005 to 2006 prices), about GBP 0.4 billion higher than expenditure on those without dementia (Henderson 2007). This was estimated as equating to approximately GBP 34,200 per person per annum for those without dementia and GBP 40,300 per person per annum for people with dementia (Henderson 2007).
Description of the intervention
The provision of high‐quality care following hip fracture has been identified as a major clinical need in the UK and elsewhere. This has been exemplified in the UK through the development of national guidelines (NICE 2017), the introduction of specific financial incentives for high‐quality care through the 'Best Practice Tariff' (NICE 2017), and the national audit of standards of care provision to this population through the National Hip Fracture Database (www.nhfd.co.uk/). For all people with hip fracture, initial management is usually provided in an acute hospital setting, where the person undergoes an operation for their hip fracture, and rehabilitation in the form of specialist orthopaedic and nursing care, physiotherapy, and occupational therapy. Best practice currently includes shared orthopaedic and geriatric (sometimes termed 'ortho‐geriatric') care pre‐ and postoperatively to ensure that recipients are medically fit for surgery and to monitor and manage any postoperative issues that may develop, such as delirium, pneumonia, anaemia, dehydration, pressure sores, or cardiovascular complications (Dy 2012; Jameson 2012). During the initial hip fracture admission or index admission (Drummond 2005), health professionals such as nurses, pharmacists, occupational therapists, physiotherapists, social workers, and dietitians may be involved in the patient's rehabilitation and care (Kammerlander 2010; Stenvall 2012). Depending on their home circumstances and postoperative functional capabilities, patients may be discharged directly to their usual residence, with or without community or outpatient rehabilitation, or may be transferred to an inpatient rehabilitation unit to receive continued multiprofessional rehabilitation. The person will remain in this rehabilitation setting until they are sufficiently independent to be discharged to their pre‐admission residence or, if this is not achievable, they may be provided with residential or nursing home care (Hashmi 2004).
There have been advances in the management of people with hip fracture over the past 15 years (Cameron 2000; Dy 2012). The notion of 'usual care' after hip fracture has changed so that a greater emphasis on postoperative physiotherapy and occupational therapy, interdisciplinary working, and integrated care packages has become standard. Research reports and subsequent clinical guidelines have recommended a number of interventions to improve outcomes for this group of patients (Chartered Society of Physiotherapy 2018; NICE 2017). These have included specific medical management by an ortho‐geriatrician on specified hip fracture wards, considered to enhance interdisciplinary team working; improvement of communication between health and social agencies (Kammerlander 2010; Stenvall 2012); provision of dedicated functional rehabilitation interventions across acute hospital and community rehabilitation settings (Al‐Ani 2010; Huusko 2000); monitoring of postoperative complications including pressure sores (Söderqvist 2007); and optimisation of nutritional levels (Hershkovitz 2010). Specific strategies proposed for people with dementia following hip fracture have included enhanced rehabilitation and care pathways, with an emphasis on orientation to the environment, cues, reminiscence and structured, familiarised routines (Strömberg 1999). Such interventions can be delivered in a variety of healthcare and domiciliary settings.
How the intervention might work
Interventions that have been proposed to improve the rehabilitation and recovery of people with dementia after hip fracture share many elements with those which have been advocated to improve outcomes for all older people after hip fracture, such as better communication between healthcare professionals and provision of wider healthcare expertise than may be conventionally found on an orthopaedic ward or in a rehabilitation setting (Söderqvist 2007). The overall effectiveness of such enhanced, multidisciplinary rehabilitation and care models remains uncertain even for people who are not cognitively impaired. A Cochrane Review was limited by considerable heterogeneity between trials, but suggested better short‐term functional outcomes for people who had enhanced, multidisciplinary rehabilitation after hip fracture (Handoll 2009). People with dementia, who have greater and more complex needs, may gain the most from these enhanced rehabilitation strategies following hip fracture surgery. Alternatively, it is possible that their more complex needs render the interventions less effective than in an elderly population without cognitive impairment. Specifically targeted additional elements and resources, drawing on best practice dementia care, may be necessary for people with dementia, and have been recommended (Söderqvist 2007).
Why it is important to do this review
More than three‐quarters of a million people in the UK have dementia (Alzheimer's Society 2014), and one in four National Health Service (NHS) beds is usually occupied by someone with dementia (Alzheimer's Research UK 2018). Fractured hips and falls are the most common reasons for hospital admission (Gill 2017). People with dementia who sustain a hip fracture have more complications, disabilities, and social needs, and hence more complex healthcare needs (Gill 2017). Whilst there have been previous reviews of rehabilitation following hip fracture, no reviews of randomised controlled trials have specifically assessed which features of rehabilitation and care are more effective for those who also have dementia. Because this population has particularly complex care needs and makes a major demand on healthcare services, this focused review of the literature was warranted.
Factors such as depression, motivation, pain, and cognitive impairment have been cited as negatively impacting on clinical outcomes in this population (Lenze 2007). Pain has been acknowledged as a particular problem which, if not assessed and managed adequately, can produce negative postoperative outcomes and complications (Feldt 1998; Morri 2018; Morrison 1998). These factors may adversely impact on the ability of a person to return to functional independence; the discharge destination; the length of their inpatient hospital stay; and rehabilitation requirements. The resulting negative consequences have a health economic impact at a personal and a societal level. People who sustain a hip fracture and have dementia experience longer hospitalisations with poorer outcomes, including higher mortality and morbidity rates, with a greater risk of requiring nursing home placement and poorer functional recovery (Gruber‐Baldini 2003; Liu 2018; Magaziner 1990; Steiner 1997). However, whilst various interventions have been supported for the targeted rehabilitation of people with dementia who experience a hip fracture (Al‐Ani 2010; Huusko 2000), these are more expensive than conventional postoperative management (Lenze 2007). More evidence is needed on the relationship between the processes and outcomes of postoperative care, length of stay, and costs in the general population of people with hip fracture, Hunt 2009, and in particular in the subpopulation of those with dementia (Henderson 2007). Decisions as to whether to allocate limited health and social care resources to these new interventions can be informed by economic evaluation, the comparative analysis of outcomes, and the costs of alternative treatment programmes (Drummond 2005).
No reviews have specifically assessed the impact of different care models on behavioural, cognitive, or other dementia‐related outcomes for people with dementia following hip fracture, nor on the relationship between these outcomes and resource use and costs. This review also aimed to examine these important questions.
Objectives
(a) To assess the effectiveness of models of care including enhanced rehabilitation strategies designed specifically for people with dementia following hip fracture surgery compared to usual care.
(b) To assess for people with dementia the effectiveness of models of care including enhanced rehabilitation strategies that are designed for all older people, regardless of cognitive status, following hip fracture surgery compared to usual care.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, quasi‐randomised (a method of allocating participants to a treatment that is not strictly random, e.g. by hospital number), or cluster‐randomised controlled clinical trials published in any language, evaluating the effectiveness for people with dementia of any model of enhanced care and rehabilitation following hip fracture surgery compared to usual care.
Types of participants
We included people who were aged 65 years or over, had any form of dementia, and had undergone hip fracture surgery for a proximal femoral fracture. We excluded trials where over 30% of participants presented with a mid‐shaft or distal femoral fracture. We used two approaches for the definition of dementia: (1) we included trials where all participants had dementia diagnosed using a validated instrument such as the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV) or International Classification of Diseases 10th revision (ICD‐10) (American Psychiatric Association 1994; World Health Organization 2015); (2) we also included trials where all participants were described as presenting with cognitive impairment that is likely to be due to dementia (e.g. persistent cognitive impairment rather than temporary, such as delirium, and not attributed to other causes such as stroke or head injury). This was termed 'probable dementia'. We considered this to be closer to the way in which people may be identified for an intervention in clinical practice. We contacted corresponding authors for further information if the method of diagnosing dementia or identifying persistent cognitive impairment was not stipulated in the original paper. Participants could have been resident in the community, in care homes, or in hospitals for short‐ or long‐term care. We included only those trials/subgroups where all participants were described as having dementia or were cognitively impaired, that is where data on the cognitively impaired subgroups were either reported separately or were available from the authors.
Types of interventions
We wanted to identify any trial that compared a control intervention consisting of usual care (including conventional rehabilitation) in the context where the trial was conducted, and an active intervention consisting of any model of care that involved enhanced rehabilitation intended to improve outcomes for elderly people after hip fracture surgery.
To meet both of our objectives, we included two types of active intervention: (1) for objective 1, the active intervention was any model of care including enhanced rehabilitation designed specifically for people with dementia. Elements in addition to usual care could have included postoperative recovery on a specialist ward, involvement of specialist staff or enhanced rehabilitation with respect to: orientation to the environment, cues, reminiscence, structured routines, or any other element drawn from dementia care practice; (2) for objective 2, the care model was intended for all older people after hip fracture surgery and designed without regard to cognitive status. In comparison to usual care, it might have included protocols for interdisciplinary working, more structured and protocol‐driven care and discharge planning, enhanced monitoring for complications that may impact on recovery, intensive rehabilitation regimens or extension of rehabilitation into the community after discharge.
Interventions could be delivered in acute hospital environments, community health or rehabilitation centres, community centres or non‐health settings, or in people's homes and residences (domiciliary).
Types of outcome measures
The primary and secondary outcomes are presented below.
Primary outcomes
Health‐related quality of life assessed using validated outcome measures such as the 36‐item Short Form Health Survey (SF‐36) (Ware 1992), Bath Assessment of Subjective Quality of Life in Dementia (BASQID) (Trigg 2007), DEMQOL (Smith 2005), 12‐item Short Form Health Survey (SF‐12) (Ware 1996), EuroQol (EQ)‐5D (EuroQol Group 1990), and Health Utility Index instruments (Feeny 2002).
Activities of daily living and functional performance assessed by validated outcome measures such as the Barthel Index (Mahoney 1965), Nottingham Extended Activities of Daily Living Scale (Nouri 1987), Oxford Hip Score (Dawson 1996), the Bristol Activities of Daily Living Score (BADLS) (Bucks 1996), or a timed walk test.
Secondary outcomes
Cognitive function as assessed using validated outcome measures such as the Alzheimer's Disease Assessment Scale Cognitive Subscale (ADASCOG) (Rosen 1984), Mini‐Mental State Examination (MMSE) (Folstein 1975), Abbreviated Mental Test (Hodkinson 1972), Addenbrooke’s Cognitive Examination Revised (ACE‐R) (Mathuranath 2005), Montreal Cognitive Assessment (MoCA) (Nasreddine 2005), Hopkins Verbal Learning Test (HVLT‐R) (Brandt 1991), or the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (Jorm 1989).
Behaviour assessed using validated outcome measures such as the Neuropsychiatric Inventory (NPI), Cummings 1994, or Cohen‐Mansfield Agitation Inventory (CMAI), Cohen‐Mansfield 1986.
Pain from any cause using validated outcome methods suited to people with dementia, such as the Pain Assessment in Advanced Dementia (PAINAD) (Warden 2003).
All‐cause mortality.
Adverse events such as deep vein thrombosis, pressure sores, pneumonia, and unplanned return to theatre.
Use of health and social care resources: hospital length of stay, hospital readmissions, discharge destination (to pre‐injury setting, residential or nursing home care), use of primary and community care support services including general physician (GP) visits, medications and tests prescribed, and community and residential rehabilitation.
Costs of hospitalisation, hospital readmission, health and social care support in the community or in residential or nursing home care, and costs to people with dementia who have had a hip fracture and to their carers (such as travel, carers' lost productivity).
Search methods for identification of studies
We performed the search in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group Specialised Register, up to and including 16 October 2019.
ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains dementia and cognitive improvement trials identified from the following.
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), PsycINFO, and LILACS (Latin American and Caribbean Health Science Information database).
Monthly searches of a number of trial registers: metaRegister of Controlled Trials, UMIN (Japan's trial register), World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/) (which covers the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov), ISRCTN, Chinese Clinical Trial Register, German Clinical Trials Register, Iranian Registry of Clinical Trials, the Netherlands National Trials Register, plus others).
Quarterly search of the Central Register of Controlled Trials (CENTRAL) in the Cochrane Library.
Monthly searches of a the grey literature source ISI Web of Science ‐ Core collection.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS website.
We ran additional separate searches in many of the above sources to ensure that our results were up‐to‐date. The search strategies used for the retrieval of reports of trials are shown in Appendix 1.
We placed no restrictions on the search with respect to date of publication, risk of bias, or language of publication.
Searching other resources
We reviewed the reference lists of all potentially eligible papers. We also asked the corresponding authors of each included paper to review the search results to identify any papers not initially found in the previous searches.
We searched the conference proceedings and abstracts from the British Orthopaedic Association Annual Congress, the European Federation of National Associations of Orthopaedics and Traumatology (EFORT), the British Hip Society, and British Trauma Society meetings. We accessed these through The Bone & Joint Journal Orthopaedic Proceedings (www.bjjprocs.boneandjoint.org.uk/). We additionally searched the British Library Database of Conference Proceedings and Journals (www.bl.uk/collection‐guides/electronic‐collections).
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Selection of studies
Two review authors (TS and AG) independently reviewed the titles and abstracts of each citation identified by the search strategy. We retrieved the full‐text version of each potentially eligible trial, and these were assessed independently for eligibility. All full‐text papers that satisfied the eligibility criteria were included in the review. The two review authors (TS and AG) discussed any disagreements about trial eligibility, referring any unresolved issues to a third review author (CF). We asked study corresponding authors to provide clarification regarding eligibility when this remained uncertain after full‐text review.
Data extraction and management
Two review authors (TS and AG) independently extracted data from the original publication(s) of each included trial. Data were recorded on a pre‐designed data extraction form. We extracted the following data: country of origin, publication date, number of participants receiving each intervention, gender, age and dementia diagnosis for participants, classification or type of femoral fracture, fracture fixation method, interval between fracture and surgical management, setting, description of control and experimental intervention, duration of intervention, follow‐up period, outcome measurements used, and results for each intervention group.
The review authors (TS and AG) resolved any disagreements on data extraction through discussion, consulting a third review author (CF) for adjudication where necessary. We tabulated all agreed‐upon descriptive data into a single document in Review Manager 5 (Characteristics of included studies) (Review Manager 2014).
Assessment of risk of bias in included studies
We evaluated the quality of the included trials and their risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2017). We assessed the following 'Risk of bias' domains for each included trial: sequence generation; allocation concealment; blinding; completeness of outcome data; selective outcome reporting; and other potential sources of bias. For each domain, we assessed whether there was a low risk of bias (if the trial matched the criteria); a high risk of bias (if the trial did not match the criteria); or an unclear risk of bias (due to under‐reporting).
Two review authors (TS and AG) independently conducted 'Risk of bias' assessments, resolving any disagreements on scoring through discussion and recourse to a third review author (AS).
We used the GRADE approach to assess the overall certainity of the evidence for each outcome. This considers risk of bias as well as imprecision in the results, inconsistency between trials, publication bias, and indirectness of the evidence.
Measures of treatment effect
For dichotomous data, we expressed the treatment effect as an odds ratio (OR) with 95% confidence interval (CI). When no event occurred for both groups (i.e. when both groups had no events), these data were reported narratively and not included in the meta‐analysis due to the risk that this may lead to a large confidence interval. For continuous data, we expressed the treatment effect as a mean difference (MD) or standardised mean difference (SMD) with their 95% CI.
Unit of analysis issues
The individual participant was the unit of analysis.
Dealing with missing data
We contacted study corresponding authors regarding any missing data from trial reports included in the review. If data remained unavailable, this was acknowledged. We did not impute missing outcome data for any outcomes or use intention‐to‐treat data where trial authors had imputed any missing data. If trial authors had imputed any missing data, we requested per‐protocol data for the review.
Assessment of heterogeneity
We evaluated clinical and statistical heterogeneity between trials. We assessed clinical heterogeneity by examining the data extraction tables. Two review authors (TS and AG) independently examined the tables and made a judgement regarding between‐trial variability with respect to the following: diagnosis, age, fracture characteristics, interventions (pre‐ and postsurgical), outcome measures, time of outcome measurement, and other aspects of study design.
We assessed statistical heterogeneity using the I² and Chi² statistics. If I² was > 50% and Chi² P > 0.10, we downgraded the certainty of the evidence (using GRADE) due to inconsistency.
Assessment of reporting biases
Too few trials were available to permit the use of funnel plots to assess risk of publication bias.
Data synthesis
Based on our evaluations of clinical heterogeneity between trials, two review authors (TS and AG) independently decided whether data from different trials were suitable for pooling in meta‐analyses. Where trials were insufficiently similar to permit pooling, we summarised the treatment effects narratively. If we considered trials sufficiently similar, we performed a meta‐analysis using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Data were insufficient to conduct our planned subgroup analyses based on age, type of dementia, or setting in which the intervention was provided. However, there were sufficient data to undertake a subgroup analysis of Huusko 2000 data on mortality and residential placement at three and 12 months postoperatively by severity of cognitive impairment.
Sensitivity analysis
We planned to conduct sensitivity analyses to explore the influence of the following factors:
The risk of bias: the analysis of data with the exclusion of results from studies which demonstrated a high risk of bias based on the Cochrane Collaboration’s risk of bias tool (Higgins 2017).
The analysis of data solely from published, peer‐reviewed papers.
We did not conduct these prespecified sensitivity analyses due to the limited meta‐analyses and similarities in the quality of evidence from the included trials.
'Summary of findings' tables
We used the GRADE approach to assess the overall certainity of evidence for specific outcomes that included pooled data (Schünemann 2011b). We downgraded the evidence from 'high certainty' by one level for serious (or by two levels for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias, as recommended by Cochrane (Schünemann 2011a).
We employed the GRADE approach to interpret findings, Langendam 2013, and used GRADEpro GDT, GRADEPRO, to import data from Review Manager 5, Review Manager 2014, to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall certainity of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes considered.
When pooled data were reported, the following outcomes were included in the 'Summary of findings' tables for each comparison.
Health‐related quality of life
Activities of daily living and functional performance
Cognitive function
Behaviour
Pain
Mortality
Adverse events (including infection, thrombosis, falls)
These outcomes include the review's primary outcome and what we considered to be the key secondary outcomes, taking into account the core outcome set for hip fracture presented in Haywood 2014. Where multiple time points were reported for an outcome, we presented the 12‐month outcome.
Results
Description of studies
For further details, see Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The results of the search are summarised in Figure 1. For this update, we identified a total of 6511 citations from the electronic search strategy and a further 21 from a search of the reference lists of potentially relevant papers. We screened 1956 records after removal of duplicates, of which 51 were deemed to be potentially eligible. We acquired the full‐text versions to evaluate them against the predefined eligibility criteria (Smith 2013). Following this, 42 papers did not satisfy the eligibility criteria. Nine papers describing seven trials satisfied the inclusion criteria and were subsequently included in the review. Two trials each reported findings in two papers (Shyu 2012; Stenvall 2012). The updated search in October 2019 identified two new trials, Freter 2017; Wyller 2012, and two ongoing trials, Dautel 2019; Hammond 2017 (see Characteristics of ongoing studies tables).
1.
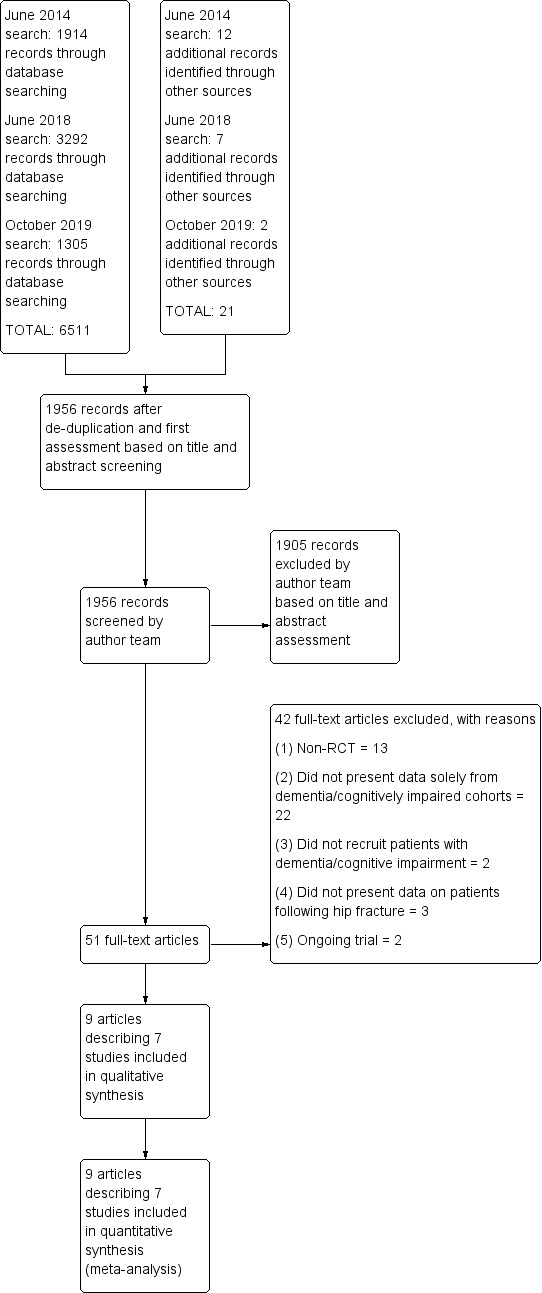
PRISMA flow diagram.
Included studies
From the seven included trials, 555 participants (282 in the experimental groups and 273 in the usual care groups) were included in the analyses in this review. We did not identify any trials that investigated the effectiveness of an enhanced rehabilitation strategy or care model specifically designed for people with dementia/cognitive impairment following hip fracture. All trials presented data from subgroups of larger randomised controlled trials of enhanced rehabilitation and care models for older people in general following hip fracture. Only two trials prespecified their analysis of the subgroup with cognitive impairment or dementia (Huusko 2000; Wyller 2012). Data from six trials were taken from papers that presented only findings related to the subgroups of people with cognitive impairment/dementia (Freter 2017; Huusko 2000; Shyu 2012; Stenvall 2012; Wyller 2012; Uy 2008). One paper presented the results of the full trial, as well as the subgroup of their participants categorised as cognitively impaired or with dementia (Marcantonio 2001).
Five included trials were funded by non‐industry funding sources (Freter 2017; Marcantonio 2001; Shyu 2012; Stenvall 2012; Wyller 2012); Huusko 2000 was funded through both industry and non‐industry funding sources; and Uy 2008 did not state their source of funding.
Participant characteristics
Diagnosis: Only one trial included participants with dementia diagnosed using a validated diagnostic instrument: Stenvall 2012 determined a diagnosis of dementia with the DSM‐IV (American Psychiatric Association 1994). One trial included participants with a diagnosis of dementia based on "expert opinion" (Wyller 2012). In practice, one specialist in geriatric medicine and one specialist in old age psychiatry independently assessed whether participants fulfilled the ICD‐10 criteria for dementia. The other five trials used various means of assessing the severity of cognitive impairment to identify participants with probable dementia. Moderate or severe impairment on the Short Portable Mental Status Questionnaire (SPMSQ), Pfeiffer 1975, was used in one trial (Uy 2008); a score of four or more on the Blessed Dementia Rating Scale, Blessed 1968, in one trial (Marcantonio 2001); and criteria based on the MMSE in two trials (Huusko 2000; Shyu 2012). Freter 2017 assessed probable dementia by determining whether participants had been previously diagnosed with dementia from a medical record review and family interview.
Age: The mean ages reported for participants were very similar across trials and intervention groups: 78 years in Marcantonio 2001 to 85 years in Wyller 2012.
Hip fracture management: Three trials presented the method of surgical management for participants with dementia (Huusko 2000; Uy 2008; Wyller 2012). Four trials did not specify the surgical fixation method for participants with dementia (Freter 2017Marcantonio 2001; Shyu 2012; Stenvall 2012).
Comorbidities: Only Stenvall 2012 reported their cohort's comorbidities on admission. Those most commonly reported were depression (n = 40), cardiovascular disease (n = 37), previous cardiovascular respiratory disease (n = 19), diabetes (n = 13), previous hip fracture (n = 11), and cancer (n = 7). Three trials measured the frequency of comorbidities using the Charlson Comorbidity Index (Charlson 1987; Marcantonio 2001; Uy 2008; Wyller 2012). Neither Marcantonio 2001 nor Wyller 2012 provided Charlson Comorbidity Index data specifically for their participants with dementia. Uy 2008 reported that both treatment groups presented with a Charlson Comorbidity Index of one at baseline assessment. Freter 2017 reported a mean of seven comorbid diseases in both intervention groups (Freter 2017).
Residential background: Four trials reported the usual residence of their participants prior to hip fracture (Huusko 2000; Stenvall 2012; Uy 2008; Wyller 2012). The majority of participants in Stenvall 2012 lived in residential, nursing, or hospital institutions before their hip fracture. In Huusko 2000, all participants were living independently in the community prior to their hip fracture. Uy 2008 reported that all their participants were nursing home residents prior to their hip fracture. In Wyller 2012, 32% of participants treated in the ortho‐geriatric ward and 30% treated in the orthopaedic care ward lived in institutional care settings before admission.
Interventions
The seven included trials presented data on enhanced rehabilitation and care models designed for all older people following hip fracture and not specifically for people with dementia. Full details on the experimental and usual care rehabilitation programmes of the included trials are provided in the Characteristics of included studies tables. We grouped the experimental interventions into the following three categories.
Enhanced interdisciplinary inpatient rehabilitation and care models (Freter 2017; Stenvall 2012; Uy 2008).
Enhanced interdisciplinary inpatient and home‐based rehabilitation and care models (Huusko 2000; Shyu 2012).
Geriatrician or ortho‐geriatrician‐led inpatient management (compared to orthopaedic‐led management) (Marcantonio 2001; Wyller 2012).
As shown in the Characteristics of included studies table, the three types of intervention all include heightened surveillance for common postoperative complications following hip fracture in older people, namely pressure sores, poor nutrition, embolic events, pneumonia, and delirium. The interdisciplinary team interventions in five trials involved staff training and strong communication across multidisciplinary teams that included geriatricians, nursing staff, physiotherapists, social workers, and psychologists (Freter 2017; Huusko 2000; Shyu 2012; Stenvall 2012; Uy 2008). Care planning and discharge liaison was also featured across these interventions. The focus of the intervention in Freter 2017 and Marcantonio 2001 was to reduce and manage delirium and acute confusion during the hospital stay. The major difference between the Freter 2017, Huusko 2000, and Shyu 2012 trials versus the Stenvall 2012 and Uy 2008 trials was that the former included continuing community rehabilitation after hospital discharge, whereas the latter made no provision for continuing rehabilitation outside hospital. As shown in the Characteristics of included studies table, the control intervention in each trial was a standard nursing, medical, and therapy intervention, identified as usual care.
Outcome measures
All outcome measures and timings of outcome assessment for the seven trials are provided in the Characteristics of included studies table. The duration of follow‐up varied across trials. In Freter 2017 and Marcantonio 2001, participants were followed up only until acute hospital discharge as the focus of these trials was on the prevention of postoperative delirium. Four trials specified the follow‐up duration after randomisation, which was four months in Uy 2008 and 12 months in Huusko 2000, Stenvall 2012, and Wyller 2012. The longest follow‐up was 24 months post‐hospital discharge in Shyu 2012.
Primary outcome measures
No included trials presented data on health‐related quality of life.
Four trials assessed activities of daily living and functional performance. Stenvall 2012 assessed walking ability using the Swedish version of the Clinical Outcome Variables, Seaby 1989, and performance of activities of daily living (ADL) using the Staircase of ADLs including the Katz Index of Independence in Activities of Daily Living (Katz 1963; Sonn 1996), which measures both personal/primary ADLs and instrumental ADLs. Shyu 2012, Uy 2008, and Wyller 2012 assessed ADLs using the Barthel Index (Mahoney 1965). Shyu 2012 also assessed the recovery of walking ability using the Chinese Barthel Index. Uy 2008 assessed mobility using a timed 2.44‐metre walk (Guralnik 2000). Wyller 2012 also assessed function using the Nottingham Extended ADL Index (NEADL) (Gladman 1993), ability to mobilise on the second postoperative day, and the Short Physical Performance Battery (SPPB) (Guralnik 2000). Mobility was recorded for a subgroup in Wyller 2012 using the activPAL, an accelerometer that was worn during the day to determine daily mobility.
Secondary outcome measures
One trial, Wyller 2012, assessed cognitive function 12 months after surgery using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (Jorm 1994).
No included trials presented data on behaviour or pain.
Five trials assessed mortality (Freter 2017; Huusko 2000; Shyu 2012; Stenvall 2012; Wyller 2012). All five provided mortality data at 12 months. Shyu 2012 also reported mortality at 24 months. Wyller 2012 also reported inpatient mortality.
Six trials assessed adverse events (Freter 2017; Huusko 2000; Marcantonio 2001; Shyu 2012; Stenvall 2012; Wyller 2012). Stenvall 2012 reported the incidence of all postoperative complications, and Shyu 2012 reported the occurrence of falls. Huusko 2000 assessed complications at three and 12 months postoperatively. Delirium was assessed and reported using various methods. Marcantonio 2001 reported cumulative incidence of delirium during an acute hospital period. Wyller 2012 assessed delirium using the Confusion Assessment Method (CAM) once daily preoperatively and until the fifth postoperative day (for all participants) or until discharge (for those with delirium) (Inouye 1990). Freter 2017 assessed delirium during the first five postoperative days using the MMSE and CAM (Folstein 1975; Inouye 1990).
A variety of measures were reported to evaluate the use of health and social care resources across six trials (Freter 2017; Huusko 2000; Marcantonio 2001; Shyu 2012; Stenvall 2012; Wyller 2012). These included analysis of length of hospital stay (Freter 2017; Huusko 2000; Marcantonio 2001; Wyller 2012), length of rehabilitation and nursing care recovery (Stenvall 2012), hospital readmissions (Shyu 2012; Stenvall 2012; Wyller 2012), accident and emergency (emergency room) visits (Shyu 2012), and discharge destination (Huusko 2000; Marcantonio 2001; Shyu 2012). Two trials reported on number of prescribed drugs used (Stenvall 2012; Uy 2008). Stenvall 2012 reported the difference between groups in the number of people in institutional care.
None of the included trials directly examined the costs of hospitalisation, hospital readmission, health and social care support, residential or nursing home care, or costs to the person with dementia or their carers (such as travel, carers' lost production).
Excluded studies
We excluded 42 papers after full text review (Figure 1) (see Characteristics of excluded studies). Our reasons for exclusion were as follows:
13 papers were not randomised controlled trials (Adunsky 2003a; Arinzon 2010; Deschodt 2011; Flikweert 2014; Heruti 1999; Horgan 2003; Jensen‐Dahm 2016; McGilton 2009; Morrison 2000; Penrod 2004; Reguant 2019; Rolland 2004; Seitz 2011a);
22 papers did not provide specific data on participants with dementia or cognitive impairment (Berggren 2019; Cameron 1993; Chong 2013; Crotty 2003; Crotty 2019; Cunliffe 2004; Espaulella 2000; Kalisvaart 2005; Karlsson 2016; Kennie 1988; Lima 2016; Martín‐Martín 2014; Moseley 2009; Naglie 2002; Oldmeadow 2006; O’Halloran 2016; Pitkala 2006; Sherrington 1997; Stenvall 2007; Strömberg 1999; Vidan 2005; Williams 2017);
three papers did not provide specific data on participants who had sustained a hip fracture (Bongartz 2017; Hauer 2017; Schwenk 2014);
two trials did not recruit participants with dementia or cognitive impairment (Mangione 2005; Mangione 2010).
Ongoing studies
We identified two ongoing trials (see Characteristics of ongoing studies) (Dautel 2019; Hammond 2017).
Risk of bias in included studies
A summary of the 'Risk of bias' assessment for each of the included trials is shown in Figure 2 and Figure 3.
2.
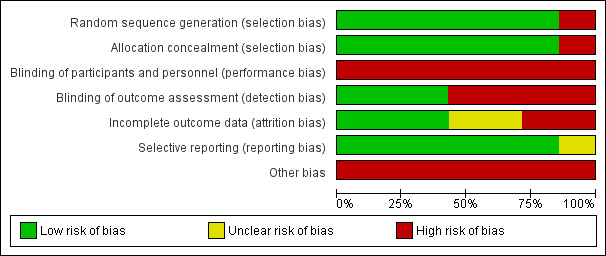
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed six trials as at low risk of selection bias with respect to random sequence generation (Huusko 2000; Marcantonio 2001; Shyu 2012; Stenvall 2012; Uy 2008; Wyller 2012). These trials clearly described their randomisation procedure, allowing replication of their allocation strategy. Six trials clearly demonstrated that allocation was concealed using sealed envelopes (Huusko 2000; Marcantonio 2001; Shyu 2012; Stenvall 2012; Uy 2008; Wyller 2012). We judged Freter 2017, which used a quasi‐randomisation approach, to be at high risk of selection bias. The researchers in this study assigned one orthopaedic ward in a tertiary care hospital as control and one ward as intervention; patients were admitted to one floor or the other from the emergency department based solely on bed availability. The authors stated that "the central assumption of randomisation (that allocation is by chance so participants cannot influence it) was not violated" (Freter 2017).
Blinding
All seven included trials were at high risk of performance bias because it was not possible to blind participants and clinicians to a recovery programme in which they were actively participating.
We judged three trials to be at low risk of detection bias (Marcantonio 2001; Uy 2008; Wyller 2012). The remaining four trials did not blind outcome assessors to participants' group allocation, thus we considered them to be at high risk of detection bias (Freter 2017; Huusko 2000; Shyu 2012; Stenvall 2012).
Incomplete outcome data
We judged three trials as at low risk of attrition bias (Freter 2017; Marcantonio 2001; Shyu 2012); all participants who had been enrolled into the trials were included in the analyses, with no loss to follow‐up. We assessed two trials that did not report loss to follow‐up as at unclear risk of attrition bias (Huusko 2000; Uy 2008). Finally, we considered two trials with relatively high rates of loss to follow‐up as at high risk of attrition bias (Stenvall 2012; Wyller 2012).
Selective reporting
We judged six trials to have a low risk of reporting bias as there was no evidence of unreported outcomes (Freter 2017; Huusko 2000; Shyu 2012; Stenvall 2012; Uy 2008; Wyller 2012). We judged one trial to be at unclear risk of reporting bias because it was unclear whether the composite measure used to identify the incidence of delirium was prospectively defined (Marcantonio 2001).
Other potential sources of bias
All seven included trials presented data from subgroups of larger trials (Freter 2017; Huusko 2000; Marcantonio 2001; Shyu 2012; Stenvall 2012; Uy 2008; Wyller 2012). With these small samples, there was a potential for baseline imbalance that could have influenced the interpretation of the intervention effect. Huusko 2000 and Stenvall 2012 reported baseline imbalances. In Huusko 2000, there was a baseline imbalance in MMSE score, with a lower median MMSE score in the experimental group. In Stenvall 2012, there was a baseline imbalance in mobility: 49% of participants in the control group had been independently mobile indoors prior to their fracture compared with 21% in the experimental group. It was not possible to assess for potential baseline imbalance in Marcantonio 2001, since these data were not presented. We considered there to be a high risk of contamination bias for six trials (Freter 2017; Marcantonio 2001; Shyu 2012; Stenvall 2012; Uy 2008; Wyller 2012). In these trials, both intervention and control rehabilitation and care models were delivered in the same hospitals, therefore staff treating control group participants could potentially have been aware of the experimental intervention, which could have influenced their practice. In Huusko 2000, intervention and control participants were rehabilitated in different hospitals, therefore we considered the risk of contamination bias to be low.
Effects of interventions
See: Table 1; Table 2; Table 3
Enhanced rehabilitation and care models designed specifically for people with dementia following hip fracture surgery
We found no trials investigating enhanced rehabilitation strategies and care models designed specifically for people with dementia following hip fracture surgery.
Enhanced rehabilitation and care models designed for all older people regardless of cognitive status following hip fracture surgery
We divided the interventions in the included trials into three types based on the nature of the experimental intervention and the setting or settings in which it was delivered. We have presented results separately for trials investigating each type of intervention.
Enhanced interdisciplinary inpatient rehabilitation and care models versus conventional inpatient rehabilitation and care models
We identified three trials comparing enhanced interdisciplinary inpatient care models with conventional usual care and for which data for participants with dementia or cognitive impairment were reported separately (Freter 2017; Stenvall 2012; Uy 2008). Uy 2008 was a very small trial and was incompletely reported; the only data we were able to extract for meta‐analysis was related to mortality. The findings for this comparison are summarised in Table 1. For Freter 2017, we used data on delirium, mortality, adverse events, health resource use (length of stay), and cognitive function that we received directly from the authors.
Health‐related quality of life
No data were presented on health‐related quality of life.
Activities of daily living and functional performance
Two trials assessed functional performance (Stenvall 2012; Uy 2008). Because of imprecision in the results, we are uncertain whether the enhanced interdisciplinary care model in Stenvall 2012 affected the following outcomes: personal ADL independence at four‐month (odds ratio (OR) 4.14, 95% confidence interval (CI) 0.40 to 42.66, 1 trial, n = 54) or 12‐month follow‐up (OR 4.62, 95% CI 0.18 to 119.63, 1 trial, n = 47); walking independence without an aid or assistance at four‐month (OR 7.63, 95% CI 0.83 to 70.53, 1 trial, n = 54) or 12‐month follow‐up (OR 7.20, 95% CI 0.74 to 70.42, 1 trial, n = 47). We assessed the evidence as of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Uy 2008 reported "non‐significant trends” for improvement in the Barthel Index and the timed walking test at one month and four months in the experimental group. However, these results were based on only three participants in the experimental group and seven participants in the control group. We considered this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Cognitive function
No data were presented on cognitive function.
Behaviour
No data were presented on behaviour.
Pain
No data were presented on pain.
All‐cause mortality
Three trials assessed mortality (Freter 2017; Stenvall 2012; Uy 2008). Because of imprecision in the results, we are uncertain as to whether the enhanced interdisciplinary care model affected mortality (OR 0.60, 95% CI 0.17 to 2.13, 3 trials, n = 152, Analysis 1.1). We considered the evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
1.1. Analysis.
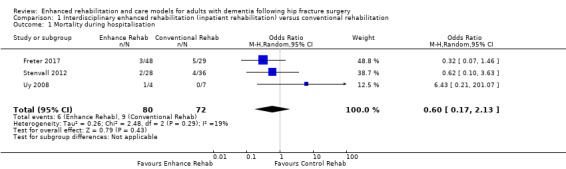
Comparison 1 Interdisciplinary enhanced rehabilitation (inpatient rehabilitation) versus conventional rehabilitation, Outcome 1 Mortality during hospitalisation.
Adverse events
Stenvall 2012 itemised the number of participants who experienced a postoperative adverse event during their inpatient hospital stay. Because of imprecision in the results, we are uncertain of the effect of the intervention on adverse events, including: pneumonia (OR 2.04, 95% CI 0.32 to 13.13, 1 trial, n = 64); decubital ulcers (OR 0.36, 95% CI 0.09 to 1.48, 1 trial, n = 64); and postoperative fracture (OR 0.17, 95% CI 0.01 to 3.39, 1 trial, n = 64). The frequency of the following adverse events was reduced in the enhanced interdisciplinary rehabilitation care model group compared to the usual care model group: urinary tract infection (OR 0.15, 95% CI 0.05 to 0.48, 1 trial, n = 64); nutritional problems (OR 0.27, 95% CI 0.08 to 0.88, 1 trial, n = 64); and recurrent falls (OR 0.00, 95% CI 0.00 to 0.03, 1 trial, n = 64). We considered the evidence for all these outcomes to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Freter 2017 reported adverse events as collective events rather than by specific complication. We could not be certain of any effect of enhanced inpatient intervention on collective adverse events (OR 0.79, 95% CI 0.31 to 1.99, 1 trial, n = 77). We also considered this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Stenvall 2012 and Freter 2017 both reported rates of postoperative delirium during hospitalisation. There may have been an important effect of enhanced interdisciplinary inpatient interventions compared to conventional care models in reducing rates of postoperative delirium during hospitalisation (OR 0.04, 95% CI 0.01, 0.22, 2 trials, n = 141, Analysis 1.2). We rated the certainity of the evidence for this outcome as low due to serious concerns about risk of bias and imprecision.
1.2. Analysis.
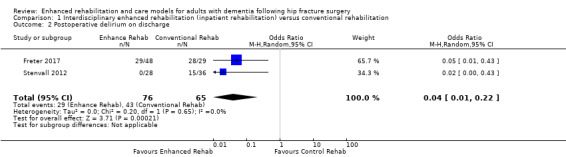
Comparison 1 Interdisciplinary enhanced rehabilitation (inpatient rehabilitation) versus conventional rehabilitation, Outcome 2 Postoperative delirium on discharge.
Use of health and social care resources
Two trials assessed length of stay in hospital (Freter 2017; Stenvall 2012). Because of imprecision in the result, we are uncertain as to whether there is an effect of the enhanced compared to the conventional rehabilitation intervention on length of stay (mean difference (MD) −5.33 days, 95% CI −16.09 to 5.44, 2 trials, n = 141, Analysis 1.3). We considered this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
1.3. Analysis.
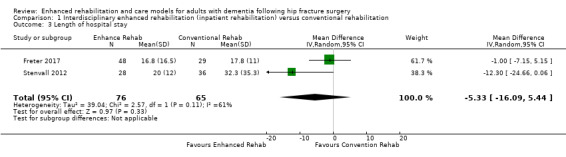
Comparison 1 Interdisciplinary enhanced rehabilitation (inpatient rehabilitation) versus conventional rehabilitation, Outcome 3 Length of hospital stay.
We are also uncertain of any effect of the enhanced rehabilitation model in Stenvall 2012 on the number of people living in institutional settings at four months (OR 1.25, 95% CI 0.31 to 5.06, 1 trial, n = 54) or 12 months (OR 0.41, 95% CI 0.06 to 2.73, 1 trial, n = 47). We considered this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Costs of hospitalisation, hospital readmission, health and social care support in the community
No data were presented on costs of hospitalisation, hospital readmission, or health and social care support in the community.
Enhanced interdisciplinary inpatient and home‐based rehabilitation and care models versus conventional rehabilitation and care models
Two trials compared enhanced interdisciplinary inpatient and home‐based rehabilitation and care models to usual care for people with dementia following hip fracture surgery (Huusko 2000; Shyu 2012). The findings for this comparison are summarised in Table 2.
Health‐related quality of life
No data were presented on health‐related quality of life.
Activities of daily living and functional performance
Shyu 2012 detected better ADL performance using the Chinese Barthel Index (0‐to‐100‐point scale, where higher scores indicate greater functional performance) in the enhanced interdisciplinary rehabilitation and care model group than in the conventional care group at three months (MD 18.81, 95% CI 9.40 to 28.22, 1 trial, n = 43) and 12 months (MD 25.40, 95% CI 10.89 to 39.91, 1 trial, n = 36). There was uncertainty about the direction of effect at 24 months (MD 7.92, 95% CI −9.88 to 25.72, 1 trial, n = 30).
Shyu 2012 also reported data on the frequency of participants regaining their pre‐fracture walking capability. They reported that a greater proportion of participants randomised to the enhanced interdisciplinary rehabilitation and care models regained pre‐fracture walking levels at three months (OR 5.10, 95% CI 1.29 to 20.17, 1 trial, n = 43) and 12 months (OR 58.33, 95% CI 3.04 to 1118.19, 1 trial, n = 36). There was uncertainty about the direction of effect at 24 months (OR 3.14, 95% CI 0.68 to 14.50, 1 trial, n = 43).
We considered the certainty for both of these outcomes at all time points to be very low due to very serious and serious concern about imprecision and risk of bias, respectively.
Cognitive function
No data were presented on cognitive function.
Behaviour
No data were presented on behaviour.
Pain
No data were presented on pain.
All‐cause mortality
We conducted meta‐analyses for mortality at three and 12 months. Because of imprecision in the results, we are uncertain as to whether the enhanced interdisciplinary care model affected mortality at three months (OR 1.20, 95% CI 0.36 to 3.93, 2 trials, n = 184, Analysis 2.1) or 12 months (OR 1.07, 95% CI 0.47 to 2.45, 2 trials, n = 177, Analysis 2.2). We considered the overall certainty for this outcome to be very low due to very serious and serious concern about imprecision and risk of bias, respectively.
2.1. Analysis.
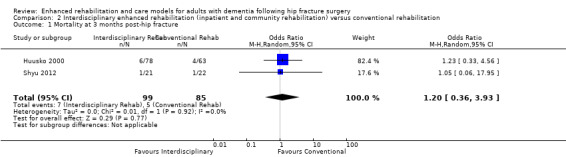
Comparison 2 Interdisciplinary enhanced rehabilitation (inpatient and community rehabilitation) versus conventional rehabilitation, Outcome 1 Mortality at 3 months post‐hip fracture.
2.2. Analysis.
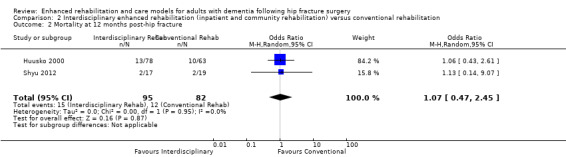
Comparison 2 Interdisciplinary enhanced rehabilitation (inpatient and community rehabilitation) versus conventional rehabilitation, Outcome 2 Mortality at 12 months post‐hip fracture.
Huusko 2000 divided their participants by severity of cognitive impairment, with severe described as a score on the MMSE of between zero and 11; moderate between 12 and 17; and mild between 18 and 23. It was possible to analyse the mortality data from Huusko 2000 in these subgroups. The results mirrored the principal analysis. We are uncertain as to whether the intervention has an important effect on mortality at three or 12 months post‐hip fracture for subgroups of participants with any severity of cognitive impairment.
Adverse events
Shyu 2012 reported the incidence of falls in each group. Because of imprecision in the results, it was not possible to determine any effect of the intervention at three months (OR 2.35, 95% CI 0.38 to 14.47, 1 trial, n = 43); 12 months (OR 0.20, 95% CI 0.01 to 4.47, 1 trial, n = 36); or 24 months (OR 0.77, 95% CI 0.16 to 3.74, 1 trial, n = 30). We considered the certainty for all of these outcomes to be very low due to very serious and serious concern about imprecision and risk of bias, respectively.
Use of health and social care resources
Huusko 2000 presented the median and range of hospital length‐of‐stay data for participants with mild, moderate, or severe dementia, as defined above. The median length of hospital stay for participants with mild dementia was 29 days (range 16 to 138 days) in the enhanced care group and 46 days (range 10 to 368 days) in the usual care group; for participants with moderate dementia, 47 days (range 10 to 365 days) and 147 days (range 18 to 365 days); and for participants with severe dementia, 85 days (range 13 to 365 days) and 67 days (range 15 to 365 days), respectively. For participants with both mild and moderate severe cognitive impairment, the median length of stay in hospital was shorter for those randomised to the enhanced care group than for those in the conventional care group (Mann‐Whitney U Test: mild dementia P = 0.002, 1 trial, n = 77; moderate dementia P = 0.04, 1 trial, n = 36). There was no significant effect of the intervention on hospital length of stay for people with severe cognitive impairment (Mann‐Whitney U Test: P = 0.902, 1 trial, n = 28). We considered the certainty for all these outcomes to be very low due to very serious and serious concern about imprecision and risk of bias, respectively.
Based on data from Huusko 2000, participants allocated to the enhanced interdisciplinary rehabilitation and care models were less likely to be living in institutional care at three months (OR 0.46, 95% CI 0.22 to 0.95, 1 trial, n = 141), but there was no clear effect at 12 months (OR 0.90, 95% CI 0.40 to 2.03, 1 trial, n = 141). No participants in Shyu 2012 lived in institutional care at these time points. We considered the certainty for this outcome to be very low due to very serious and serious concern about imprecision and risk of bias, respectively.
Based on data from Shyu 2012, there was no clear evidence of an effect of the intervention on the following outcomes: frequency of hospital admissions (three months: 0 admissions; 12 months: OR 0.71, 95% CI 0.10 to 4.86, 1 trial, n = 43; 24 months: OR 1.00, 95% CI 0.14 to 7.10, 1 trial, n = 43); and attendance at the emergency room/accident and emergency (three months: OR 0.50, 95% CI 0.04 to 5.97, 1 trial, n = 43; 12 months: OR 0.50, 95% CI 0.04 to 5.97, 1 trial, n = 36; 24 months: OR 3.79, 95% CI 0.17 to 86.13, 1 trial, n = 30). We considered the certainty for all of these outcomes to be very low, reflecting serious concerns about risk of bias and very serious concerns about imprecision of point estimates.
We were able to perform a subgroup analysis of data from Huusko 2000 for residential placement at three and 12 months by MMSE grouping to assess the impact of severity of cognitive impairment on this outcome. There was an effect on residential placement, with 15 people (63%) with moderate dementia in the enhanced interdisciplinary rehabilitation and care model group still living independently at three months compared to two (17%) in the usual care group (OR 8.33, 95% CI 1.48 to 46.94, 1 trial, n = 36). This effect was not clearly maintained at 12 months (OR 3.33, 95% CI 0.78 to 14.31, 1 trial, n = 36). For those participants with mild dementia, 32 people (91%) in the enhanced interdisciplinary rehabilitation and care model group were living independently three months postoperatively compared to 28 (67%) in the usual care group (OR 5.33, 95% CI 1.39 to 20.49, 1 trial, n = 77). Again, this effect was not maintained 12 months postoperatively (OR 1.05, 95% CI 0.36 to 3.015, 1 trial, n = 77). There was no clear evidence of a difference in place of residence for people with severe dementia at three months (OR 0.73, 95% CI 0.15 to 3.65, 1 trial, n = 28) or 12 months postoperatively (OR 1.17, 95% CI 0.22 to 6.20, 1 trial, n = 28). We considered this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Costs of hospitalisation, hospital readmission, health and social care support in the community
No data were presented on costs of hospitalisation, hospital readmission, or health and social care support in the community.
Geriatrician‐led inpatient management versus orthopaedic‐led inpatient management
Two trials compared clinical outcomes of an experimental care model involving geriatrician/ortho‐geriatrician‐led management to a model of usual care in which management was led by an orthopaedic surgeon (Marcantonio 2001; Wyller 2012). The findings for this comparison are summarised in Table 3.
Health‐related quality of life
No data were presented on health‐related quality of life.
Activities of daily living and functional performance
Wyller 2012 assessed ADLs and functional performance. There was no evidence of a difference in performance of ADLs when assessed using the Bristol Activities of Daily Living Score (BADLS) (0 to 60 points, where higher scores equate to poorer performance) at either four months (MD −1.20, 95% CI −3.20 to 0.80, 1 trial, n = 112) or 12 months (MD −1.50, 95% CI −3.92 to 0.92, 1 trial, n = 87), nor when assessed using the Nottingham Extended ADL Index (NEADL) (0 to 22 points, where higher scores equate to better performance) at four months (MD −1.70, 95% CI −5.96 to 2.56, 1 trial, n = 112) or 12 months (MD −3.00, 95% CI −8.11 to 2.11, 1 trial, n = 87). There was also no effect when functional performance was measured using the Short Physical Performance Battery (SPPB) (0 to 12 points, where higher scores equate to better performance) at four months (MD 0.10, 95% CI −0.77 to 0.97, 1 trial, n = 112) or 12 months (MD 0.30, 95% CI −0.65 to 1.25, 1 trial, n = 87). We judged the evidence for this outcome to be of very low certainty due to serious concerns regarding risk of bias and serious or very serious concerns regarding imprecision.
Cognitive function
One trial assessed cognitive function measured using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (1 to 5 points, where higher scores equate to poorer cognitive function) between geriatrician‐led management and orthopaedic‐led management groups (Wyller 2012). There was no important effect on cognitive function at four months (MD 0.10, 95% CI −0.09 to 0.29, 1 trial, n = 112) or 12 months postsurgery (MD 0.10, 95% CI −0.18 to 0.38, 1 trial, n = 87). We considered this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Behaviour
No data were presented on behaviour.
Pain
No data were presented on pain.
All‐cause mortality
Only Wyller 2012 reported inpatient mortality. The result was imprecise, and we were uncertain about any difference between groups during admission (OR 1.56, 95% CI 0.25 to 9.58, 1 trial, n = 162) or at four months (OR 1.31, 95% CI 0.60 to 2.86, 1 trial, n = 112) or 12 months postsurgery (OR 2.00, 95% CI 0.74 to 5.36, 1 trial, n = 87). We considered this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Adverse events
Both trials reported incidence of delirium during the period of acute hospitalisation (Marcantonio 2001; Wyller 2012). There was no evidence of an important effect of the intervention on delirium during hospitalisation, but the result was based on a small number of events and was imprecise (OR 0.94, 95% CI 0.52 to 1.72, 2 trials, n = 212, Analysis 3.1). We judged this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
3.1. Analysis.
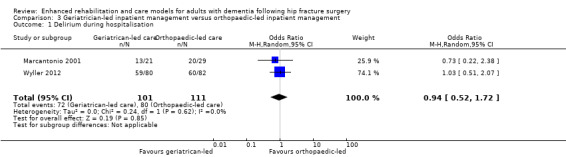
Comparison 3 Geriatrician‐led inpatient management versus orthopaedic‐led inpatient management, Outcome 1 Delirium during hospitalisation.
Use of health and social care resources
Geriatrician‐led management in Marcantonio 2001 may have reduced length of hospital stay compared to orthopaedic surgeon‐led management (MD 4.00 days, 95% CI 3.61 to 4.39, 1 trial, n = 162). We judged this evidence to be of low certainty due to serious concerns about risk of bias and imprecision.
Wyller 2012 reported health resource use (new nursing home admissions). The results were imprecise, so we could not be certain about any effect on this outcome at either four months (OR 1.35, 95% CI 0.55 to 3.35, 1 trial, n = 112) or 12 months (OR 1.03, 95% CI 0.38 to 2.74, 1 trial, n = 87). We considered this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Costs of hospitalisation, hospital readmission, health and social care support in the community
Wyller 2012 reported data on hospital readmissions. Results were imprecise, and we were uncertain of any effect on hospital readmission at four months (OR 0.53, 95% CI 0.18 to 1.56, 1 trial, n = 112) or 12 months (OR 1.14, 95% CI 0.34 to 3.87, 1 trial, n = 87). We considered this evidence to be of very low certainty due to very serious and serious concern about imprecision and risk of bias, respectively.
Discussion
Summary of main results
We found seven trials examining enhanced rehabilitation and care models for older people following a hip fracture that presented data on those participants who had dementia or cognitive impairment at baseline. Five trials compared enhanced interdisciplinary rehabilitation and care models (in‐hospital, or both in‐hospital and at home) with usual care. Based on these five trials, there was low‐certainty that enhanced care and rehabilitation in‐hospital may lead to a lower incidence of postoperative delirium. There was very low‐certainty for all other outcomes, including activities of daily living and functional performance. Two trials compared the outcomes of geriatrician‐led care with usual care led by an orthopaedic surgeon. There was low‐certainty that geriatrician‐led management may lead to fewer days in‐hospital. Again, the certainty for other outcomes was very low.
Overall completeness and applicability of evidence
The trials included in this review have highlighted the considerable uncertainty that remains surrounding the evidence for enhanced interdisciplinary rehabilitation and care models for people with dementia following a hip fracture above usual active rehabilitation and conventional care models. The literature was incomplete in a number of important aspects. Firstly, no tested intervention had been specifically designed for people with hip fracture and dementia. The available trials were subgroup analyses from larger randomised controlled trials that assessed the outcomes of enhanced care models for older people following hip fracture surgery. Consequently, the included trials were not based on sample size calculations for this group and therefore lacked power to detect a statistically significant difference, even if one exists (type 2 statistical error) for people with dementia. A possible consequence of only testing generic rehabilitation models, as opposed to those specifically for people with dementia, is that interventions such as orientation techniques, environment, cues, reminiscence, and structured, familiarised routines have yet to be investigated for this hip fracture population.
The primary outcomes of this review were functional performance and health‐related quality of life. Only four trials measured functional performance (Shyu 2012; Stenvall 2012; Uy 2008; Wyller 2012), and no trial assessed health‐related quality of life. There was limited assessment of cognitive function postintervention, which is unsurprising considering that the trials were designed for all older people and not specifically for those with dementia. A number of outcomes of interest to us were not reported, including the assessment of behaviour and pain. These outcomes have been previously acknowledged as difficult to assess in people with dementia and cognitive impairment (Hebert‐Davies 2012). Some specific instruments have been developed, including the Neuropsychiatric Inventory (NPI) to assess behaviour (Cummings 1994), and the Pain Assessment in Advanced Dementia (PAINAD) to evaluate pain in this population (Warden 2003). There was also limited assessment of the use of health and social care resources and costs. This was a major limitation to the completeness of the literature and a consideration for future trials in rehabilitation and care models for people with dementia.
The literature presents outcomes from programmes of enhanced rehabilitation and care that are context‐specific, so that the effectiveness of the individual components of these is unknown. Questions remain, including determining the effect on postoperative recovery of being in a specialist ortho‐geriatric ward; the dose, frequency, duration, and intensity of physiotherapy and occupational therapy; the effectiveness of targeted and structured reminiscence therapy; the adoption of familiarised routines; and the addition of assistive technologies. Furthermore, the impact on effectiveness and resource use of delivering interventions in different settings (acute hospital, community health or rehabilitation centres, or non‐health settings) and delivery by different personnel (qualified healthcare professionals, social care providers, or informal caregivers) is not known. Finally, due to the limited amount of data, it remains unclear how important participant factors such as age, comorbid diseases including frailty, and type or stage of dementia are to the outcome of specific management strategies.
Quality of the evidence
Using GRADE methods, we rated the certainty of the outcomes as low or very low, reflecting serious concerns about risk of bias in the included data and very serious concerns about imprecision of the results. This grading means that we are very uncertain about the estimates of effect. Accordingly, the current evidence base is insufficient in both size and quality. The 'Risk of bias' tool identified two key recurrent limitations across the included trials: not blinding participants and clinical/research personnel, and not blinding assessors to group allocation (Figure 3). Whilst it is logistically difficult, if not impossible, to blind participants and clinical/research team members to group allocation during study participation or delivery of a physical intervention, assessor blinding would have been possible in these trial designs. This may have prevented detection bias from impacting on the results of the trials, and must be considered in future trials of rehabilitation and care models. Since all included trials were subgroup analyses, there were important baseline imbalances (for severity of cognitive impairment in Huusko 2000 and for pre‐fracture mobility in Stenvall 2012), which may have impacted on the estimated intervention effect in an unpredictable way.
As highlighted previously, the trials were not designed to identify differences in outcome for participants with dementia. The numbers of participants with dementia recruited to these trials was not based on a power calculation, and hence there was a lack of power to detect a difference in outcome between groups, even if one exists. This may account for the non‐statistically significant differences reported for the majority of outcomes in the included trials and the imprecision of our effect estimates.
Finally, the included trials diagnosed dementia inadequately, with only Stenvall 2012 specifically stating that dementia was formally assessed by a geriatrician using the DSM‐IV tool. Huusko 2000 provided sufficient evidence through their report and through personal communication that their cohort consisted of people with dementia, excluding other causes of cognitive impairment. However, they only specifically evaluated cognitive impairment using a single severity tool, the MMSE, rather than a physician‐based dementia diagnosis. This was also the case for Shyu 2012. Dementia was diagnosed by severity of cognitive impairment using the Short Portable Mental Status Questionnaire (SPMSQ) in Uy 2008 and the Blessed Dementia Rating Scale in Marcantonio 2001. Finally, Wyller 2012 diagnosed dementia based on physician "expert opinion", whilst Freter 2017 determined a dementia diagnosis through medical record review to identify a previous diagnosis and based on family interview. To facilitate generalisability to specific populations, it is critical that formal tools and assessment procedures are undertaken to correctly categorise people with or without dementia. However, it is recognised that many people with dementia may be undiagnosed, and the adoption of a pragmatic point‐of‐admission tool to identify cognitive impairment, such as the MMSE, may be applicable to provide a surrogate for dementia. This tension between generalisability to specific populations and pragmatism on diagnosis should be considered in future research.
Potential biases in the review process
This review was designed to minimise the risk of potential biases. Strategies to address this included searching a number of the most relevant published and unpublished literature databases on health and social care rehabilitation and medicine to limit selection bias and identify all relevant trials. Secondly, two review authors independently evaluated studies for inclusion and performed data extraction and 'Risk of bias' assessments to minimise the risk of inaccurate reporting of trial findings.
Because of the small number of trials, it was not possible to construct a funnel plot to assess the risk of small‐trial effects which might indicate publication bias. It is likely that other trials of generic rehabilitation strategies after hip fracture have included participants with dementia but have not published separate data on these participants.
Agreements and disagreements with other studies or reviews
The conclusions drawn from this review do not agree with the conclusions of the original trials included in this review. This can be attributed to the interpretation of data following the 'Risk of bias' assessment, which provides a more cautious analysis of the findings.
Four systematic reviews have assessed general management strategies for people with dementia following hip fracture surgery (Allen 2012; Chu 2016; Menzies 2010; Resnick 2016). All four reviews identified the same trials included in this review, in addition to a number of non‐randomised controlled trials. Chu 2016 and Resnick 2016 included Moseley 2009, which we excluded from our review because data on between‐group difference were not specifically reported for participants with cognitive impairment/dementia.
Whilst these systematic reviews only searched published literature databases, their conclusions agree with those of this review in that no trials have reported the effectiveness of dementia‐specific interventions for rehabilitation of people with dementia following hip fracture surgery. All four reviews were in agreement with this review in that certainty was limited due to a number of major weaknesses. However, these systematic reviews supported the use of enhanced interdisciplinary rehabilitation and the use of protocol‐driven geriatric care, particularly for people with mild to moderate dementia (Allen 2012; Menzies 2010); yet no review emphasised that when this was compared to an active treatment and usual intervention, this apparent difference was largely clinically or statistically insignificant. Three reviews stated that the current evidence base should be interpreted cautiously (Allen 2012; Chu 2016; Resnick 2016), providing some agreement that there is insufficient research to ascertain the optimal rehabilitation and recovery pathway for people with dementia following hip fracture surgery, most notably for people with moderate to severe dementia and those who reside in institutional care homes.
Authors' conclusions
Implications for practice.
Whilst enhanced rehabilitation strategies (inpatient) and geriatrician‐led recovery may offer some benefits over conventional rehabilitation and recovery for people with dementia following hip fracture surgery, the certainty of the evidence is low or very low. There is currently insufficient evidence to inform the adoption of enhanced interdisciplinary rehabilitation and care models specifically for people with dementia following hip fracture surgery over usual, conventional rehabilitation and care models. The optimal rehabilitation and care model for this population is unclear. Existing randomised controlled trials have not assessed strategies intended to improve quality of life and reduce cognitive deterioration in this population. It is therefore unknown whether care and rehabilitation models are more effective if they include dementia‐focused interventions such as provision of cues, reminiscence therapy, the adoption of familiarised routines, or the use of assistive technologies.
Implications for research.
This review has highlighted a number of priorities that should be considered in the design of future research. Firstly, given the uncertainty regarding the optimal enhanced rehabilitation and care model for people with dementia following hip fracture surgery, research is required to assess the clinical effectiveness of different models. This may include investigating care models with differing intensities, frequencies, durations, and locations for physiotherapy, occupational therapy, and other rehabilitative expertise. Additionally, assessing the delivery of these interventions in different locations (hospital, outpatients/community and home settings) and care provision by different health and social care workers or carers and family would provide valuable information to understand how best to rehabilitate this population.
No trials have assessed the cost‐effectiveness of different enhanced rehabilitation and care models. Whilst four trials assessed hospital length of stay (Freter 2017; Huusko 2000; Marcantonio 2001; Stenvall 2012), this is a challenging measure to interpret. A reduction in length of stay may be related to an increased rate of institutionalisation or be a function of an enhanced discharge pathway as part of an enhanced rehabilitation pathway. Considering other measures of cost‐effectiveness is therefore warranted. Furthermore, the assessment of quality of life (including for family members), pain, and behavioural outcomes is warranted. Finally, although challenging, including people with severe cognitive impairment is important, so that this group of the dementia population is investigated in future trials. Strategies to include this group in future research should be developed to better understand whether and how a more inclusive approach for dementia research can be achieved.
What's new
| Date | Event | Description |
|---|---|---|
| 16 October 2019 | New citation required but conclusions have not changed | Two new studies were added and the content revised and updated in line with MECIR. Conclusions unchanged. |
| 16 October 2019 | New search has been performed | The most recent search was performed on 16 October 2019. |
Acknowledgements
We thank Jenny McCleery and Sue Marcus for their assistance in the preparation of the protocol and the final review, and Anna Noel–Storr for her assistance in developing and conducting the search strategy for the review. We would like to thank Dr Yea‐Ing Shyu, Chang Gung University, Taiwan; Dr Lars Strömberg, Red Cross University College, Sweden; Professor Ian Cameron, The University of Sydney, Australia; Dr Susan Freter, Dalhousie University, Halifax, Canada; and Dr Leiv Otto Watne, University of Oslo, Norway for reviewing the search results and for their helpful comments.
We would like to thank peer reviewers Jacqueline Close and Stephen Kates and consumer reviewer Cathie Hofstetter for their comments and feedback
Appendices
Appendix 1. Sources searched and search strategies from 2014 to 2019
| Source | Search strategy | Hits retrieved |
| ALOIS (www.medicine.ox.ac.uk/alois) [Date of most recent search: 16 Oct 2019] |
hip OR fracture OR surgery OR operation OR femur OR femoral | June 2014: 120 June 2018: 3 Oct 2019: 126 |
| Cochrane Bone, Muscle and Trauma Group Specialised Register [Date of most recent search: 16 Oct 2019] |
hip OR fracture OR surgery OR operation OR femur OR femoral | June 2018: 220 (all dates) Oct 2019: 127 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) [Date of most recent search: 16 Oct 2019] [Note: for the June 2018 top‐up search, a new term was added and searched across all dates: geriatric* assess*. This term identified 994 results from Medline] |
1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. exp Femur/ 22. exp Fractures, Bone/ 23. exp Fracture Fixation/ 24. exp Fracture Healing/ 25. or/22‐24 26. 21 and 25 27. (hip or hips or pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular*).ti,ab. 28. ((femur* or femoral*) adj3 (neck or proximal)).ti,ab. 29. 27 or 28 30. ((hip or hips or pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular* or ((femur* or femoral*) adj3 (neck or proximal))) adj4 fracture).ti,ab. 31. geriatric* assess*.ti,ab. 32. randomized controlled trial.pt. 33. controlled clinical trial.pt. 34. randomi?ed.ab. 35. randomly.ab. 36. placebo.ab. 37. drug therapy.fs. 38. trial.ab. 39. groups.ab. 40. ("double‐blind*" or "single‐blind*").ti,ab. 41. (RCT or CCT).ti,ab. 42. or/32‐41 43. (animals not (humans and animals)).sh. 44. 41 not 42 45. 29 or 30 46. 20 and 44 and 45 |
June 2014: 255 June 2018: 1145 Oct 2019: 123 |
| EMBASE 1980‐2018 June 25 (Ovid SP) [Date of most recent search: 16 Oct 2019] [Note: for the June 2018 top‐up search, a new term was added and searched across all dates: geriatric* assess*. This term identified 674 results from Embase] |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. femur/ or femur fracture/ 25. fracture/ 26. 24 and 25 27. (hip or hips or pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 28. fracture*.ti,ab. 29. femur.ti,ab. 30. geriatric* assess*.ti,ab. 31. or/24‐29 32. 23 and 31 33. randomized controlled trial/ 34. trial.ab. 35. randomly.ab. 36. groups.ab. 37. randomi?ed.ti,ab. 38. placebo.ab. 39. RCT.ti,ab. 40. "double‐blind*".ti,ab. 41. or/33‐40 42. 32 and 41 |
June 2014: 716 June 2018: 1029 Oct 2019: 426 |
| PsycINFO 1806‐June week 3 2018 (Ovid SP) [Date of most recent search: 16 Oct 2019] [Note: for the June 2018 top‐up search, a new term was added and searched across all dates: geriatric* assess*. This term identified 32 results from PsycINFO] |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. (hip or hips or pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular*).ti,ab. 26. fracture*.ti,ab. 27. femur.ti,ab. 28. femoral*.ti,ab. 29. geriatric* assess*.ti,ab. 30. or/25‐28 31. 24 and 30 32. exp Clinical Trials/ 33. randomly.ab. 34. randomi?ed.ti,ab. 35. RCT.ti,ab. 36. groups.ab. 37. placebo.ab. 38. "double‐blind*".ti,ab. 39. or/32‐38 40. 31 and 39 |
June 2014: 86 June 2018: 203 Oct 2019: 15 |
| CINAHL (EBSCOhost) [Date of most recent search: 16 Oct 2019] |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX hip OR hips OR fracture* OR femur OR femoral OR pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular* S21 (MH "Hip Fractures") S22 S20 OR S21 S23 S19 AND S22 S24 (MH "Randomized Controlled Trials") OR (MH "Clinical Trials") S25 TX randomly S26 AB trial S27 AB placebo S28 AB placebo S29 AB "double‐blind*" S30 AB groups S31 AB groups S32 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 S33 S23 AND S32 |
June 2014: 125 June 2018: 107 Oct 2019: 95 |
| ISI Web of Science (1945‐present) and conference proceedings [Date of most recent search: 16 Oct 2019] |
Topic=(dement* OR alzheimer* OR "lewy bod*" OR DLB OR "vascular cognitive impairment*" OR FTD OF FTLD OR "cerebrovascular insufficienc*") AND Topic=(hip OR hips OR fracture* OR femur OR femoral OR pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular*) AND Topic=(randomly OR trial OR cluster* OR RCT OR placebo OR randomised OR randomized) Timespan=All years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, CCR‐EXPANDED, IC. |
June 2014: 324 June 2018: 217 Oct 2019: 76 |
| LILACS (BIREME) [Date of most recent search: 16 Oct 2019] |
cadera OR hip OR hips OR caderas OR fractura OR fracture OR fémur OR femur OR fêmur OR quadril [Words] and dementia OR demência OR alzheimer OR "cognitive impair$" OR "deterioro cognitivo" [Words] | June 2014: 12 June 2018: 22 Oct 2019: 1 |
| CENTRAL (The Cochrane Library) (Issue 6 of 12, 2018) [Date of most recent search: 16 Oct 2019] |
#1 MeSH descriptor: [Dementia] explode all trees #2 dement* #3 alzheimer* #4 lewy* near/2 bod* #5 deliri* #6 chronic near/2 cerebrovascular #7 "organic brain disease" or "organic brain syndrome" #8 "normal pressure hydrocephalus" and "shunt*" #9 "benign senescent forgetfulness" #10 cerebr* near/2 deteriorat* #11 cerebral* near/2 insufficient* #12 pick* near/2 disease #13 creutzfeldt or jcd or cjd #14 huntington* #15 binswanger* #16 korsako* #17 "cognit* impair*" #18 MeSH descriptor: [Cognition Disorders] explode all trees #19 MCI #20 ACMI #21 ARCD #22 SMC #23 CIND #24 BSF #25 AAMI #26 LCD #27 AACD #28 MNCD #29 MCD #30 "N‐MCI" or "A‐MCI" or "M‐MCI" #31 (cognit* or memory or cerebr* or mental*) near/3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*) #32 "preclinical AD" #33 "pre‐clinical AD" #34 aMCI or MCIa #35 "CDR 0.5" or "clinical dementia rating scale 0.5" #36 "GDS 3" or "stage 3 GDS" #37 "global deterioration scale" and "stage 3" #38 "mild neurocognit* disorder*" #39 prodrom* near/2 dement* #40 episodic* near/2 memory #41 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 #42 hip or hips or pertrochant* or intertrochant* or trochanteric or subtrochanteric or extracapsular* #43 (femur* or femoral*) near/3 (neck or proximal) #44 MeSH descriptor: [Femur] explode all trees #45 MeSH descriptor: [Fractures, Bone] explode all trees #46 MeSH descriptor: [Fracture Fixation] explode all trees #47 MeSH descriptor: [Fracture Healing] explode all trees #48 #42 or #43 or #44 or #45 or #46 or #47 #49 #48 and #41 in Trials |
June 2014: 148 June 2018: 316 Oct 2019: 289 |
| Clinicaltrials.gov (www.clinicaltrials.gov) [Date of most recent search: 16 Oct 2019] |
hip OR hips OR surgery OR pertrochant* OR intertrochant* OR trochanteric OR subtrochanteric OR extracapsular OR femur OR femoral | Interventional Studies | dementia OR alzheimer OR alzheimers OR lewy OR vascular cognitive impairment | June 2014: 104 June 2018: 2 Oct 2019: 17 |
| ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Date of most recent search: 16 Oct 2019] |
#1 hip AND dementia = 5 #2 fracture AND dementia = 9 #3 femur AND dementia = 10 |
June 2014: 24 June 2018: 40 Oct 2019: 10 |
| TOTAL before de‐duplication | June 2014: 1914 June 2018: 3292 Oct 2019: 1305 TOTAL: 6511 |
|
| TOTAL after de‐duplication and first‐assessment (Note: first assessment not performed for the Oct 2019) | June 2014: 296 June 2018: 581 Oct 2019: 1079 TOTAL: 1956 |
|
Data and analyses
Comparison 1. Interdisciplinary enhanced rehabilitation (inpatient rehabilitation) versus conventional rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality during hospitalisation | 3 | 152 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.13] |
| 2 Postoperative delirium on discharge | 2 | 141 | Odds Ratio (M‐H, Random, 95% CI) | 0.04 [0.01, 0.22] |
| 3 Length of hospital stay | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐5.33 [‐16.09, 5.44] |
Comparison 2. Interdisciplinary enhanced rehabilitation (inpatient and community rehabilitation) versus conventional rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at 3 months post‐hip fracture | 2 | 184 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.36, 3.93] |
| 2 Mortality at 12 months post‐hip fracture | 2 | 177 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.47, 2.45] |
Comparison 3. Geriatrician‐led inpatient management versus orthopaedic‐led inpatient management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Delirium during hospitalisation | 2 | 212 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.52, 1.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Freter 2017.
| Methods | Pragmatic (quasi‐randomised) clinical trial comparing the feasibility (adherence) and effectiveness (prevalence of delirium, length of stay, mortality, discharge site) of delirium‐friendly reprinted postoperative orders (PPOs) for individuals with hip fracture, administered by regular orthopaedic nurses, with routine postoperative orders. Trial was conducted in Canada. This paper presented data of a subgroup analysis of people with probable dementia as part of the larger randomised controlled trial. |
|
| Participants |
Numbers: 283 patients over the age of 65 Group allocation: Intervention group: 144; control group: 139. Of these, 48 participants with probable dementia were randomised to the enhanced rehabilitation and 29 participants were randomised to the control intervention. Diagnosis/cognitive status: Preoperative MMSE: 20.9 intervention group; 22.3 control group, MMSE cut‐points not described. Age: Intervention group: 83.2 years; control group: 82.5 years Gender mix: Intervention group: 30 male (21%); control group: 40 male (29%) Usual place of residence: Not reported Surgical management: The surgical procedures undertaken to manage the hip fracture were not stated. Comorbidities: Mean number of comorbidities reported: intervention group: 7.3; control group: 7.4 Eligibility: Inclusion: individuals aged 65 years and older with an admitting diagnosis of hip fracture. Exclusion criteria were pathological fracture, involvement in motor vehicle accident or multiple trauma, previous ipsilateral hip surgery, inability to understand and converse in English, non‐ambulatory pre‐fracture status, and severe acute comorbidity preoperatively (e.g. overwhelming infection, severe congestive heart failure). |
|
| Interventions |
Delirium‐friendly postoperative care: Admitted to intervention ward and provided with PPOs with delirium‐friendly options and doses for nighttime sedation, analgesia, and nausea and attention to catheter removal and bowel movements. Regular postoperative care: Admitted to control ward, not provided with PPOs |
|
| Outcomes |
Follow‐up intervals: Outcomes were collected during the hospital stay (postoperative days 1 to 5) and at discharge. No follow‐up beyond this. Outcomes of interest to this review: Delirium assessed using the CAM and MMSE; medication use (haloperidol); length of stay on the orthopaedic unit; hospital mortality; discharge destination; complications (need for transfusion, infection, requiring antibiotics, need for reoperation, falls, cardiac complications, thromboembolic complication, chronic obstructive lung disease exacerbations). |
|
| Notes |
Funding Sources: Nova Scotia Health Research Foundation Grant and Capital Health Research Fund Personal communication from S Freter regarding randomisation: "We assigned one orthopaedic ward in our tertiary care hospital as control, and one ward as intervention. Patients are admitted to one floor or the other from the emergency department based solely on bed availability. Admission to a given ward is by chance allocation, as all surgeons admit to all wards, occupancy is very high and the wards have similar numbers of beds, including private and semi‐private rooms. The central assumption of randomisation (that allocation is by chance so that the participants cannot influence it) was not violated" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "one orthopaedic ward was assigned as control and one ward as intervention. Patients were admitted to one floor or the other from the emergency department based solely on bed availability. Admission to a given ward is by change allocation, because all surgeons admit to all wards, occupancy is high, and all wards have similar numbers of beds...under these circumstances, the central assumption of randomisation (that allocation is by chance so that participants cannot influence it) is not violated." (p 568) Comment: Quasi‐randomisation approach |
| Allocation concealment (selection bias) | High risk |
Quote: "Patients were admitted to one floor or the other from the emergency department based solely on bed availability." (p 568) Comment: Not concealed allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: "It was not possible to blind research personnel to treatment group, because allocation was conducted according to floor" (p 569) Comment: Not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "It was not possible to blind research personnel to treatment group, because allocation was conducted according to floor" (p 569) Comment: Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All data accounted for at time of discharge for those who entered the trial (p 570). |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported in Methods section (p 569) appeared in the Results (p 570). |
| Other bias | High risk |
Comment: The data were a subgroup of a larger RCT. There was a risk of baseline imbalance between the groups that was not presented, and cohort was underpowered. This may have impacted negatively on the estimation of intervention effects. Comment: For the larger RCT there was a baseline imbalance in the number of participants with dementia, with a greater number in the intervention group (n = 48) compared to the control group (n = 29) (p 569). Comment: High risk of contamination bias where intervention and control care pathways were delivered in the same hospital (p 568). |
Huusko 2000.
| Methods | A randomised controlled trial comparing interdisciplinary geriatric recovery of inpatients with probable dementia following hip fracture surgery in Finland. This was a subgroup analysis of people with probable dementia as part of a larger randomised controlled trial. |
|
| Participants |
Numbers: Overall, 243 independently living people aged 65 years or older admitted to hospital with hip fracture. This included 141 people with probable dementia. Group allocation: Regarding those with probable dementia, 78 participants were randomised to the interdisciplinary intervention, 63 to the conventional recovery. Diagnosis/cognitive status: Probable dementia was determined using the assessment of cognitive impairment employing the MMSE. Severe dementia was classified as a score of 0 to 11, moderate dementia 12 to 17, and mild dementia 18 to 23. Participants with an MMSE score of 24 to 30 were classified as normal. MMSE was assessed 10 days after surgery and randomisation. In the interdisciplinary intervention group, the frequency of MMSE score was: 0 to 11: 19; 12 to 17: 24; 18 to 23: 35; 24 to 30: 41. In the conventional rehabilitation group, the frequency of MMSE was: 0 to 11: 9; 12 to 17: 12; 18 to 23: 42; 24 to 30: 56. Age: Mean age of the overall cohort was 80 years, of which 174 were women and 69 were men. No data on mean age or gender mix for the probable dementia‐specific subgroup. Usual place of residence: Not stated. Surgical management: All trochanteric fractures were managed with osteosynthesis. In the interdisciplinary intervention group, for cervical fractures, 60 participants were managed with a hemiarthroplasty, 6 with a total hip replacement, and 12 with open reduction internal fixation. In the conventional rehabilitation group, for cervical fractures, 53 participants were managed with a hemiarthroplasty, 10 with a total hip replacement, and 16 with open reduction internal fixation. No specific data were presented for the people with probable dementia. Eligibility: All participants were living independently and had been able to walk unaided before the fracture. Exclusions were people with pathological fractures, multiple fractures, serious early complications, calcitonin treatment, and terminally ill people. |
|
| Interventions |
Interdisciplinary recovery intervention: Referral to a geriatric ward. Postoperatively participants were then managed by a interdisciplinary team consisting of a geriatrician internist, a specially trained general practitioner, nurses with training in the care of older people, a social worker, a neuropsychologist, an occupational therapist, and physiotherapists. For up to 4 days each week, this was supplemented with consultant specialists in physical medicine, a neurologist, and a psychiatrist. Collaboration between the family, participant, and the interdisciplinary team was encouraged, as was communication with local health centres, nursing homes, home help, and home care. Rehabilitation interventions included provision of advice, training, encouragement, and listening to participant's concerns, drug treatment, physiotherapy, occupational therapy, speech and language therapy, and help with appliances, equipment, and daily living aids. Participants allocated to the interdisciplinary team were assessed by the geriatric team. Physiotherapy was undertaken twice daily with daily activities practised throughout the day with nurses. Weekly joint meetings between nurses and physiotherapist were undertaken to discuss methods of improving rehabilitation. Each participant was provided with a daily schedule of rehabilitation to support early ambulation, self‐motivation, and to optimise function. Walking aid appliances were reviewed by physiotherapists, whilst occupational therapists evaluated the participant's needs for activities of daily living. Communication between family/carer and participants with the nursing and physiotherapy team was provided on numerous occasions for all participants, reinforced with a hip fracture brochure. Discharge planning was undertaken in weekly team meetings with the interdisciplinary team, family, and participant. This was supplemented by a physiotherapy‐led home visit if required. All participants discharged to independent living had 10 home visits from the physiotherapist on discharge. Conventional recovery intervention: Referral to local hospital. All participants encouraged to mobilise on the first postoperative day. No further information provided. |
|
| Outcomes |
Follow‐up intervals: Point of discharge, 3 months and 12 months postsurgery Outcomes of interest to this review: Length of hospital stay; mortality; place of residence after surgery |
|
| Notes |
Funding sources: Central Finland Healthcare District, Kuopio University Hospital, Emil Aaltonen Foundation, Uulo Arhio Foundation, and Novartis Finland. Sample size powered for whole trial of people with probable dementia and cognitively intact participants (250 in total; 125 per group). The trial was not powered to compare interventions specifically for people with probable dementia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The allocation sequence was computer‐generated and sealed in numbered, opaque envelopes in Helsinki, Finland, by the information technology department of Novartis before the trial was started. The envelopes were stored on the orthopaedic ward by the head nurse until patients were randomised" (p 1108) Comment: Done |
| Allocation concealment (selection bias) | Low risk |
Quote: "The allocation sequence was computer‐generated and sealed in numbered, opaque envelopes in Helsinki, Finland, by the information technology department of Novartis before the trial was started. The envelopes were stored on the orthopaedic ward by the head nurse until patients were randomised" (p 1108) Comment: Done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: "We could not blind the staff doing interventions or assessments" (p 1108) Comment: Not done. In addition, due to the nature of the intervention, it would not be possible to blind the participants or their families/carers. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Comment: "We could not blind the staff doing interventions or assessments" (p 1108) Comment: Not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Quote: "1 participant in the intervention group and 4 in the control group were not tested with the MMSE" (p 1109) Comment: Attrition occurred. The analysis was therefore conducted on 238 participants for the whole trial (p 1109). The attrition rate for people with probable dementia is unknown. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes planned in the Methods section were reported in the Results section (pp 1108‐9). |
| Other bias | High risk | Comment: The data were a subgroup of a larger RCT. Randomisation of the whole cohort was not stratified for cognitive status, therefore there was a baseline imbalance between groups with respect to lower MMSE score in the intervention group. This may have impacted negatively on the estimation of intervention effects. |
Marcantonio 2001.
| Methods | A randomised controlled trial comparing a geriatrician‐led recovery on a general orthopaedic ward compared to an orthopaedic surgeon‐led conventional rehabilitation and recovery intervention delivered on an orthopaedic ward for inpatients following hip fracture surgery in the USA. This paper presented data of a subgroup analysis of people with probable dementia as part of the larger randomised controlled trial. |
|
| Participants |
Sample size: 126 participants were randomised to the 2 groups. Group allocation: 62 participants were randomised to receive the geriatrician‐led recovery intervention, whilst 64 participants received the orthopaedic surgeon‐led recovery intervention from the hospital ward. Diagnosis/cognitive status: From the subgroup of people with cognitive impairment, 21 participants were allocated to the geriatrician‐led recovery compared to 29 to the orthopaedic‐led recovery group. Cognitive function was assessed with the MMSE, delirium with the DSI, severity of delirium with the MDAS, and the ascertainment of delirium with the CAM. Proxy assessments were made using the Blessed Dementia Rating Scale. Pre‐fracture probable dementia was classified as a Blessed score of 4 or higher. 21 participants in the geriatrician‐led recovery group were thus classified as having probable dementia as opposed to 29 in the orthopaedic surgeon‐led recovery group. Age: The mean age of the geriatrician‐led recovery intervention group was 78 years (SD 8), as opposed to 80 years (SD 8) in the group that received the orthopaedic surgeon‐led recovery intervention in the hospital ward. Gender mix: The geriatrician‐led recovery intervention group consisted of 13 men and 49 women, whilst there were 14 men and 50 women in the orthopaedic surgeon‐led recovery intervention group from the hospital ward. Surgical management: Hip replacement surgery (unspecified if hemiarthroplasty or total hip arthroplasty) was performed in 20 participants in the geriatrician‐led recovery group and 22 participants in the orthopaedic‐led recovery group. Usual place of residence: Not stated. Comorbidites: Comorbidites were assessed using the Charlson Index. 24 participants in the geriatrician‐led recovery consultation review group and 21 participants in the orthopaedic‐led recovery group had a Charlson Index of 4 or greater. Eligibility: Inclusion: people aged 65 years and older admitted for primary surgical repair of hip fracture. Exclusion: presence of metastatic cancer or comorbid illnesses likely to reduce life expectancy to less than 6 months, or inability to obtain informed consent within 24 hours of surgery or 48 hours of admission. If patients demonstrated evidence of probable dementia or delirium at the time of enrolment, consent was also obtained from a designated healthcare proxy. |
|
| Interventions |
Geriatrician‐led recovery intervention: Geriatric consultation preoperatively or within 24 hours postoperatively. A geriatrician performed daily visits to each participant randomised to this group and made targeted recommendations based on a protocol on aspects of care including: oxygen delivery; fluid and electrolyte balance; pain management; medication review to eliminate unnecessary medications; regulation of bowel and bladder function; nutritional intake; early mobilisation and rehabilitation; prevention, early detection, and treatment of major postoperative complications such as cardiac conditions, embolism, respiratory conditions, and urinary tract infections; optimising environmental stimuli through the provision of glasses and hearing aids, and the provision of clocks, calendars, radios, tape recorders, and soft lighting; and the treatment of agitated delirium. No more than 5 recommendations could be prioritised after the initial visit, and no more than 3 after follow‐up visits. Orthopaedic‐led recovery intervention: Pre‐ and postoperative management by the orthopaedic team with reactive internal medicine or geriatric consultation rather than on a proactive basis as per the geriatrician‐led recovery group. |
|
| Outcomes |
Follow‐up intervals: Daily assessment of outcomes during acute hospital stay. Outcomes of interest to this review: MMSE; DSI; MDAS; CAM; incidence of severe delirium, defined as a CAM‐defined delirium when the MDAS score was 18 or higher on a least 1 hospital day; hospital length of stay; discharge disposition. |
|
| Notes |
Funding sources: Older Americans Independence Center and Charles Farnsworth Trust. The sample size calculation was based on a target to observe a third reduction of delirium in the intervention groups compared to usual care with an 80% power. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: Participants randomised "by opening a sealed envelope containing the randomisation assignment derived from a random number table" (p 517). Comment: Done |
| Allocation concealment (selection bias) | Low risk |
Quote: Participants randomised "by opening a sealed envelope containing the randomisation assignment derived from a random number table" (p 517). Comment: Done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Due to the nature of the interventions, it was not possible to blind participants or personnel to the intervention received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: The researcher "conducted the assessments blinded to the intervention status of the subjects" (p 517). Comment: Done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "follow‐up was completed on all randomise subjects" (p 518) Comment: Done |
| Selective reporting (reporting bias) | Unclear risk | Comment: MMSE, DSI, and MDAS were collected to inform the incidence of delirium, but were not reported as single outcomes. No trial protocol was presented to confirm full reporting of outcomes, therefore it was unclear whether this measure was prospectively defined. |
| Other bias | High risk |
Comment: This was a subgroup analysis of a larger RCT. It was not possible to assess whether there was a difference in baseline characteristics between groups. Comment: High risk of contamination bias where intervention and control care pathways were delivered in the same hospital (p 517). |
Shyu 2012.
| Methods | A randomised controlled trial comparing an interdisciplinary recovery intervention (inpatient and community) to conventional recovery for people with probable dementia following hip fracture surgery in Taiwan. | |
| Participants |
Sample size: 160 people recruited. Group allocation: Interdisciplinary rehabilitation (n = 79); conventional rehabilitation (n = 81) groups. Diagnosis/cognitive status: 24 (29.6%) in the interdisciplinary recovery intervention and 27 (34.2%) in the conventional recovery group were cognitively impaired according to MMSE. MMSE cut‐offs for differing severities of cognitive impairment were not described. Age: Mean age of participants with probable dementia was 81.3 years in the interdisciplinary recovery intervention group and 81.7 years in the conventional recovery group. Gender mix: The interdisciplinary recovery intervention group consisted of 24 women and 3 men with probable dementia, whilst the conventional recovery group consisted of 16 women and 8 men with probable dementia. Surgical management: For the whole cohort, 100 participants received an open reduction internal fixation procedure, whilst 60 participants received a hemiarthroplasty. Usual place of residence: Not stated. Eligibility: Patients were included if they were: (1) age 60 years or older; (2) admitted to hospital for an accidental single‐side hip fracture; (3) receiving hip arthroplasty or internal fixation; (4) able to perform full range of motion against gravity and against some or full resistance before hip fracture; (5) moderately dependent or better in ADLs before hip fracture (score ≥ 70 on the CBI); and (6) living in northern Taiwan. People were excluded if they were: (1) severely cognitively impaired (score < 10 on the Chinese MMSE); (2) terminally ill. Probable dementia was determined using the assessment of cognitive impairment employing the MMSE. On the basis of the pre‐discharge cognitive function assessment, participants were categorised as cognitively impaired and assigned to the cognitive‐impairment group if they had < 6 years of education and a Chinese MMSE score < 21 or had ≥ 6 years of education and scored < 25. |
|
| Interventions |
Interdisciplinary recovery intervention: The intervention programme included 3 components: a geriatric consultation service; a rehabilitation programme; and a discharge‐planning service. Each participant in this group received a geriatric consultation by a geriatrician and geriatric nurses. This assessed participants to determine potential medical and functional problems and to decrease delays preoperatively. This was used to allow the geriatric consultant to make recommendations regarding the timing of surgery, infection and thromboembolic prophylaxis, postoperative nutritional management, urinary tract management, and delirium management. Postoperatively, this preoperative assessment formed the basis of an individualised care plan for each participant, delivered by the interdisciplinary healthcare team. This team consisted of: a gerontological nurse, the geriatrician, the primary surgeon, a rehabilitation physician, geriatric nurses, and a physical therapist. Every participant in the intervention group received both in‐hospital rehabilitation (delivered during hospitalisation) and in‐home rehabilitation (delivered in the home setting). Rehabilitation started 1 day after surgery and continued until 3 months after discharge. Both rehabilitation phases consisted of a hip fracture‐oriented rehabilitation programme to restore deteriorated physical fitness. The inpatient hospital rehabilitation consisted of daily visits from the geriatric nurse and rehabilitation physician and twice‐daily visits from the physical therapist. During the in‐home rehabilitation programme, the geriatric nurse visited 4 times during the first month and 4 times during the second and third months postdischarge. Physical therapists visited 3 times postdischarge. The interdisciplinary team's discharge service was delivered by geriatric nurses and included a discharge assessment, necessary referrals, a home assessment, and suggested environmental modifications. Discharge assessment, which occurred during hospitalisation, evaluated caregiver competence, resources, family function, participant’s self‐care ability, and the need for community or long‐term care services. Conventional recovery programme: Rehabilitation was not interdisciplinary with no continuity of care between healthcare professionals or inpatient/in‐home rehabilitation. Inpatient rehabilitation consisted of 3 physical therapy sessions and no in‐home rehabilitation. No further information on the conventional recovery and rehabilitation programme was provided. |
|
| Outcomes |
Follow‐up intervals: 1, 3, 6, 12, 18, and 24 months after hospital discharge. Outcomes of interest to this review: Recovery of walking ability (comparing before and after fracture mobility) based on the Chinese Barthel Index; ability to perform ADLs based on the Chinese Barthel Index; occurrence of falls; mortality; emergency room visits; hospital readmissions; and incidence of institutionalisation to care/nursing facility. Outcomes not of interest to this review: Hip flexion ratio (range of motion of the affected hip joint divided by the range of motion of the unaffected hip joint). |
|
| Notes |
Funding sources: National Health Research Institute, Taiwan. Sample size was not based on a power calculation. Unclear how and where follow‐up data collection was performed. The trial excluded people with severe cognitive impairment, so the population from which the sample was drawn might have been less cognitively impaired than populations sampled in other trials. The findings of non‐significant differences in mortality and institutionalisation amongst older participants with and without cognitive impairment might have been due to excluding the sickest and most cognitively impaired people, who were most likely to die or to be institutionalised. The numbers of deaths and institutionalisation were thus small. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: Participants "were randomly assigned to an intervention or control group by flipping a coin" (p 532). Comment: Done |
| Allocation concealment (selection bias) | Low risk |
Quote: Randomisation was "by flipping a coin" (p 532). Comment: Done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Due to the nature of the interventions, it was not possible to blind participants or personnel to the intervention received. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: No documentation as to whether outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "data from subjects who drop out can still contribute to the estimation of parameters" (p 532) Comment: All participants who were lost to follow‐up were accounted for (Figure 1). |
| Selective reporting (reporting bias) | Low risk | Comment: All outcome measures reported in the Methods section were reported and accounted for in the Results section. |
| Other bias | High risk | Comment: High risk of contamination bias where intervention and control care pathways were delivered in the same hospital (pp 530‐1). |
Stenvall 2012.
| Methods | A randomised controlled trial comparing an interdisciplinary recovery programme (inpatient) to a conventional recovery programme for people after hip fracture surgery in Sweden. This is a subgroup analysis of people with dementia as part of a larger randomised controlled trial. |
|
| Participants |
Sample size: 64 people with dementia were analysed from a total cohort of 199. Group allocation: 28 assigned to the multidisciplinary recovery programme, 36 to the conventional recovery programme. Diagnosis/cognitive status: Assessed by a geriatrician using the DSM‐IV. Cognitive impairment was evaluated using the MMSE. Mean MMSE score at admission was 8.6 (SD 7.1) for the multidisciplinary recovery programme and 6.9 (SD 5.0) for the conventional recovery programme. MMSE cut‐points were not clearly reported. Gender mix: The cohort consisted of 47 women and 17 men. Age: Mean age of participants was 81.0 for the multidisciplinary recovery programme and 83.2 for the conventional recovery programme. Surgical management: The surgical procedures undertaken to manage the hip fracture were not stated. Usual place of residence: 22 participants (79%) in the multidisciplinary recovery programme and 26 participants (72%) in the conventional recovery programme lived in institutional care prior to hospitalisation. Comorbidites: The frequency of comorbidities was presented for the multidisciplinary recovery programme and conventional recovery programme. These were: cancer (3, 4), previous stroke (9, 10), previous hip fracture (6, 5), diagnosis of depression (15, 25), diabetes (6, 7) and cardiovascular disease (16, 21), respectively. Eligibility: Patients were included if they: (1) presented with a femoral neck fracture; (2) were aged 70 years or over; (3) were admitted to the orthopaedic department at Umeå University Hospital, Sweden. Patients were excluded if they presented with: (1) rheumatoid arthritis; (2) severe hip osteoarthritis; (3) severe renal failure; (4) pathological fracture; (4) or were bedridden pre‐fracture. |
|
| Interventions |
Multidisciplinary recovery programme: All multidisciplinary team members, consisting of a physician, nurse, and occupational therapist and physiotherapist, complied with a comprehensive geriatric assessment and rehabilitation programme. This consisted of: staff education; greater team working and communication; individualised care planning and rehabilitation; active prevention, detection, and treatment of postoperative complications, especially delirium; focused attention on improving bowel and bladder care and minimising complications; reasons for poor sleep were investigated; prevention and treatment of decubitus ulcers; a pain management programme; prescription of oxygen‐enriched air during the first postoperative day; surveillance of body temperature, blood pressure; nutritional advice and support from a dietitian; early postoperative mobilisation in the first 24 hours; rehabilitation by the physiotherapists, occupational therapist, and care staff, which was progressed daily throughout the participant's inpatient rehabilitation and focused on re‐ablement to functional return; specific assessment and management of falls and osteoporosis. The staffing ratio on the multidisciplinary recovery programme ward was 1.07 nurses/aids per bed. The multidisciplinary team assessed all participants 4 months postoperatively for postoperative complications and to determine any further care needs. Conventional recovery programme: This was delivered on a specialist orthopaedic ward, with subsequent, longer‐term follow‐up (required by 13 participants) delivered on a geriatric ward. The staffing ratio in the conventional recovery programme was 1.01 nurses/aids per bed in the orthopaedic ward, and 1.07 nurses/aids per bed in the geriatric ward. The control group followed conventional postoperative routines, which included the non‐formalised and inconsistent provision of team working, individualised care planning and rehabilitation, prevention, detection and treatment of postoperative complications (especially delirium), improving bowel and bladder care and minimising complications, reasons for poor sleep were investigated, prescription of oxygen‐enriched air during the first postoperative day, surveillance of blood pressure, nutrition, early postoperative mobilisation in the first 24 hours, rehabilitation by the physiotherapists, occupational therapist, and care staff and progressed daily throughout the participant's inpatient rehabilitation focusing on re‐ablement to functional return, and specific assessment and management of falls and osteoporosis. All participants in the conventional recovery intervention received prevention and treatment of decubitus ulcers, a pain management programme, and surveillance of body temperature, but unlike in the multidisciplinary rehabilitation programme, they were not reviewed by a dietitian regarding nutritional support. |
|
| Outcomes |
Follow‐up intervals: During hospital stay; on discharge from the hospital; at 4 months (± 2 weeks) and 12 months (± 1 month) postoperatively. Outcomes of interest to this review: Incidence of postoperative complications, readmission; inpatient hospital days after discharge; walking ability using the Swedish version of the Clinical Outcome Variables; functional performance of ADLs using the Staircase of ADL including the Katz Index of Independence in Activities of Daily Living, which measures both personal/primary ADLs and instrumental ADLs; MMSE; modified Organic Brain Syndrome Scale to assess cognitive, perceptual, emotional, and personality changes and fluctuations in clinical state; and living situation, i.e. institutionalised or independent living in a community dwelling. Outcomes not of interest to this review: the Geriatric Depression Scale to assess signs of depression. |
|
| Notes | Funding sources: Vardal Foundation, the Joint Committee of the Northern Health Region of Sweden (Visare Norr), the Swedish Dementia Foundation, the Foundation of the Medical Faculty, the University of Umeå, the County Council of Västerbotten ("Dagmar", "FoU", and "Äldrecentrum Vasterbotten"), the Swedish Research Council, and the National Society for Research on Aging (RÅF) in Sweden. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Using opaque sealed envelopes, sequentially numbered, not computer‐generated but mixed by people not involved in the trial, patients were randomly assigned to post‐operative care in a geriatric ward with a special intervention programme or to conventional care in an orthopedic ward. All participants received this envelope while in the emergency room but it remained unopened until immediately before surgery to ensure that all participants received similar pre‐operative treatment. People not involved in the trial carried out the randomisation procedure" (p 285) Comment: Done |
| Allocation concealment (selection bias) | Low risk |
Quote: "People not involved in the trial carried out the randomisation procedure" using opaque sealed envelopes, sequentially numbered." (p 285) Comment: Done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No specific blinding of participants or personnel. However, blinding could have been difficult due to the nature of the interventions (p 285). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Assessors were not blinded to group allocation (p 285). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Participant loss to follow‐up was accounted for in Figure 1 (p 286). 9 participants in the interdisciplinary inpatient rehabilitation group and 8 in the conventional rehabilitation group were lost to follow‐up. Missing data were not accounted for in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported in the Methods were accounted for and presented in the Results (pp 285‐7). |
| Other bias | High risk |
Comment: The data were a subgroup of a larger RCT. Randomisation of the whole cohort was not stratified for cognitive status. There was a baseline imbalance between groups with respect to mobility. This may have impacted negatively on the estimation of intervention effects. Comment: High risk of contamination bias where intervention and control care pathways were delivered in the same hospital (p 285). |
Uy 2008.
| Methods | A randomised controlled trial comparing clinical outcomes of an inpatient multidisciplinary rehabilitation intervention to a conventional rehabilitation for people following hip fracture who live in nursing homes in Australia. | |
| Participants |
Sample size: A total of 11 participants enrolled in the trial; 10 participants completed the 4‐month follow‐up period and were included in the analysis. Group allocation: 3 participants were randomised to the inpatient multidisciplinary rehabilitation intervention group, whilst 7 participants were randomised to the conventional rehabilitation intervention group. Diagnosis/cognitive status: All participants were classified as having moderate to severe cognitive impairment using the SPMSQ (the 'best' score within this cohort being 6). Age: Median age was 80 years in the inpatient multidisciplinary recovery intervention group and 83 years in the conventional recovery intervention group. Gender mix: All participants were women. Surgical management: In the inpatient multidisciplinary recovery intervention group, hemiarthroplasty (n = 1) and compression screw and plates (n = 2) were undertaken. In the conventional recovery intervention group, hemiarthroplasty (n = 5) and compression screw and plates (n = 2) were undertaken. Usual place of residence: 100% of the cohort lived in nursing homes prior to hospitalisation. Comorbidites: Comorbidites were assessed using the Charlson Index; the median Charlson Index for both groups was 1. Eligibility: Inclusion: women living in a nursing home within the catchment of the trial hospital prior to a hip fracture; ambulant without the assistance of another person prior to their hip fracture; able to follow commands at the time of seeking informed consent in the postoperative period. |
|
| Interventions |
Interdisciplinary intervention: Immediate postoperative nursing care plan devised to encourage early mobility and self‐care. Physician with a special interest in rehabilitation and geriatric medicine reviewed the participant with 24 hours postoperatively. This assessment was used to identify and treat intercurrent illness, review prior level of disability, and determine the participant’s level of social support. The physician planned the woman’s rehabilitation. Mobilisation began post‐check x‐ray and stable medical condition. The objective was to sit out of bed on the day after the operation and attempt walking the next day. Mobilisation was supervised by the nursing staff in consultation with a visiting physiotherapist. Mobilisation supervised by a physiotherapist was provided daily each weekday; 2 sessions of physiotherapy daily were considered to be ideal. Mobility training was continued by the nursing staff at other times. The orthopaedic surgeon and the rehabilitation physician reviewed the woman 3 or 4 times weekly.
Participants returned to their nursing home as soon as was feasible given their medical condition. The rehabilitation physician liaised with the nursing home and confirmed arrangements for the mobilisation of the participant. Mobilisation was supervised by the nursing staff in consultation with a visiting physiotherapist. Progress was checked after several weeks by the rehabilitation physician, and orthopaedic review was arranged according to need. Conventional recovery intervention: Standard treatment provided at the trial hospital at the time of the trial. Participants living in nursing homes and those with limited disability were discharged when deemed orthopaedically appropriate. |
|
| Outcomes |
Follow‐up intervals: 1 month and 4 months post‐hip fracture. Outcomes of interest to this review: Barthel Index, gait velocity measured by a timed 2.44‐metre walk test. |
|
| Notes | Funding sources: Not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "the randomisation sequence generated from a random number table was used to allocate eligible participants to an intervention group or a control group" (p 43) Comment: Done |
| Allocation concealment (selection bias) | Low risk |
Quote: "concealed randomisation using numbered opaque envelopes" (p 43) Comment: Done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No specific blinding of participants or personnel. However, blinding could have been difficult due to the nature of the interventions (p 43). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "follow‐up data were collected by a research nurse who was masked to the allocation of the trial participants" (p 43) Comment: Done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
Quote: "there was one early death in the intervention group" (p 43) Comment: It was not clear whether or not this could have been related to the trial management. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported in the Methods section (p 43) were reported in the Results section (pp 43‐4). |
| Other bias | High risk |
Comment: Due to limited trial details, it was unclear whether there were any other potential biases. There was a possible high risk of bias due to lack of power (small sample size recruited; bias due to type 2 statistical error); the trial was terminated early and failed to adequately recruit. Comment: High risk of contamination bias where intervention and control care pathways were delivered in the same hospital (p 43). |
Wyller 2012.
| Methods | A randomised controlled trial to assess the effect of a model of preoperative as well as early postoperative care, treatment, and rehabilitation in a dedicated ortho‐geriatric ward in a single‐blind randomised trial in Norway. | |
| Participants |
Number: Total of 327 participants were included. Group allocation: 162 (+ 1 erroneously sent to incorrect ward) included in ortho‐geriatric ward group, 165 (+ 1 erroneously sent to incorrect ward) included in the orthopaedic ward group. Of these, 80 participants with probable dementia were randomised to the ortho‐geriatric ward group and 82 participants with probable dementia were randomised to the control group. Diagnosis/cognitive status: Expert opinion 80 (49%) ortho‐geriatric ward, 82 (49%) orthopaedic ward. Age: 84 years (55 to 99) ortho‐geriatric ward, 85 years (46 to 101) orthopaedic ward. Gender mix: 42 (26%) male inortho‐geriatric ward, 38 (23%) male in orthopaedic ward. Usual place of residence: 52 (32%) in ortho‐geriatric ward, 50 (30%) in orthopaedic ward "living in institution". Surgical management: Ortho‐geriatric ward: hemiarthroplasty 74; osteosynthesis 88; total hip replacement 0; Girdlestone procedure 1; not operated 0. Conventional recovery intervention: hemiarthroplasty 71; osteosynthesis 91; total hip replacement 1; Girdlestone procedure 0; not operated 3. Comorbidities: Charlson Comorbidity Index score (median, IQR): ortho‐geriatric ward: 1 (0 to 2); conventional ward: 1 (0 to 2). Eligibility: Eligible participants will be admitted acutely for a femoral neck fracture, a trochanteric or a subtrochanteric femoral fracture. Patients will be excluded if: (1) hip fracture as part of multi‐trauma or high‐energy trauma (defined as a fall from a level higher than 1 metre); 1 recent fracture in addition to the hip fracture (e.g. radius or shoulder) is acceptable; (2) regarded as moribund at admittance; (3) absence of a valid informed consent or assent. |
|
| Interventions | Operative and anaesthesiologic procedures will be the same in the 2 groups. Ortho‐geriatric intervention: Intervention group participants were to be transferred as soon as possible to the ortho‐geriatric ward, stabilised there preoperatively, and transferred back to the same ward postoperatively for further treatment and rehabilitation. Conventional recovery intervention: A traditional orthopaedic ward with conventional rehabilitation. |
|
| Outcomes |
Follow‐up intervals: 4 and 12 months. Outcomes of interest to this review: A composite endpoint by the Clinical Dementia Rating (CDR) and the 10‐words memory task from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) battery; ADL Scale; NEADL scale; IQCODE assessment of cognitive function; intrahospital mortality; cumulative mortality; the Short Physical Performance Battery (SPPB) scale; pre‐/postoperative delirium; duration/severity of delirium; other complications; incidence of probable dementia 12 months postoperatively; length of hospital stay. Outcomes not of interest to this review: markers of bone turnover; micronutrients in blood. |
|
| Notes | Funding sources: Research Council of Norway, Oslo University Hospital, The Sophies Minde Foundation, The Norwegian Association for Public Health and Civitan's Research Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomisation was based on computer‐generated random numbers (blocks of variables and unknown size) and was carried out by a statistician not involved in the clinical service" (p 2) Comment: Done |
| Allocation concealment (selection bias) | Low risk |
Quote: "Allocation was by sealed opaque envelopes" (p 2) Comment: Done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Due to the nature of the interventions, it was not possible to blind participants or personnel to the intervention received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: Assessors "were blinded to allocation and delirium status during the hospital stay" (p 4). Comment: Done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 163 participants allocated to geriatric ward, data collected on 98 at 12 months; 166 participants allocated to orthopaedic ward, data collected on 95 at 12 months. |
| Selective reporting (reporting bias) | Low risk | Comment: All data in the Methods section were reported in the Results section. |
| Other bias | High risk | Comment: High risk of contamination bias where intervention and control care pathways were delivered in the same hospital (pp 2‐3). |
ADLs: activities of daily living
BI: Barthel Index
CAM: Confusion Assessment Method
CBI: Chinese Barthel Index
CMMSE: Chinese Mini‐Mental State Examination
DSI: delirium symptom interview
DSM‐IV ‐ Diagnostic and Statistical Manual of Mental Disorders, fourth edition
IQCODE ‐ Informant Questionnaire on Cognitive Decline in the Elderly
IQR – Inter‐Quartile Range
MDAS: Memorial Delirium Assessment Scale
MMSE: Mini‐Mental State Examination
NEADL ‐ Nottingham Extended Activities of Daily Living Scale
RCT – Randomised Controlled Trial
SD: standard deviation
SPMSQ: Short Portable Mental Status Questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adunsky 2003a | Non‐randomised controlled trial |
| Arinzon 2010 | Non‐randomised controlled trial |
| Berggren 2019 | Does not provide specific data on participants with dementia or cognitive impairment |
| Bongartz 2017 | Does not provide specific data on participants with hip fracture |
| Cameron 1993 | Does not provide specific data on participants with dementia or cognitive impairment |
| Chong 2013 | Does not provide specific data on participants with dementia or cognitive impairment |
| Crotty 2003 | Does not provide specific data on participants with dementia or cognitive impairment |
| Crotty 2019 | Does not provide specific data on participants with dementia or cognitive impairment |
| Cunliffe 2004 | Does not provide specific data on participants with dementia or cognitive impairment |
| Deschodt 2011 | Non‐randomised controlled trial |
| Espaulella 2000 | Does not provide specific data on participants with dementia or cognitive impairment |
| Flikweert 2014 | Non‐randomised controlled trial |
| Hauer 2017 | Does not provide specific data on participants with hip fracture |
| Heruti 1999 | Non‐randomised controlled trial |
| Horgan 2003 | Non‐randomised controlled trial |
| Jensen‐Dahm 2016 | Non‐randomised controlled trial |
| Kalisvaart 2005 | Does not provide specific data on participants with dementia or cognitive impairment |
| Karlsson 2016 | Does not provide specific data on participants with dementia or cognitive impairment |
| Kennie 1988 | Does not provide specific data on participants with dementia or cognitive impairment |
| Lima 2016 | Does not provide specific data on participants with dementia or cognitive impairment |
| Mangione 2005 | Did not recruit participants with dementia or cognitive impairment |
| Mangione 2010 | Did not recruit participants with dementia or cognitive impairment |
| Martín‐Martín 2014 | Does not provide specific data on participants with dementia or cognitive impairment |
| McGilton 2009 | Non‐randomised controlled trial |
| Morrison 2000 | Non‐randomised controlled trial |
| Moseley 2009 | Does not provide specific data on participants with dementia or cognitive impairment |
| Naglie 2002 | Does not provide specific data on participants with dementia or cognitive impairment |
| Oldmeadow 2006 | Does not provide specific data on participants with dementia or cognitive impairment |
| O’Halloran 2016 | Does not provide specific data on participants with dementia or cognitive impairment |
| Penrod 2004 | Non‐randomised controlled trial |
| Pitkala 2006 | Does not provide specific data on participants with dementia or cognitive impairment |
| Reguant 2019 | Non‐randomised controlled trial |
| Rolland 2004 | Non‐randomised controlled trial |
| Schwenk 2014 | Does not provide specific data on participants with hip fracture |
| Seitz 2011a | Non‐randomised controlled trial |
| Sherrington 1997 | Does not provide specific data on participants with dementia or cognitive impairment |
| Stenvall 2007 | Does not provide specific data on participants with dementia or cognitive impairment |
| Strömberg 1999 | Does not provide specific data on participants with dementia or cognitive impairment |
| Vidan 2005 | Does not provide specific data on participants with dementia or cognitive impairment |
| Williams 2017 | Does not provide specific data on participants with dementia or cognitive impairment |
Characteristics of ongoing studies [ordered by study ID]
Dautel 2019.
| Trial name or title | Multifactorial intervention for hip and pelvic fracture patients with mild to moderate cognitive impairment: study protocol of a dual‐centre randomised controlled trial (OF‐CARE) |
| Methods | A randomised controlled trial to compare a multifactorial transitional care intervention after inpatient rehabilitation with usual care for people who have sustained a hip or pelvic fracture and who have mild to moderate cognitive impairment. |
| Participants |
Number: Will recruit 240 hip or pelvic fracture patients admitted to the geriatric rehabilitation departments of the Robert‐Bosch‐Hospital Stuttgart and the Agaplesion Bethanien Hospital Heidelberg (both in Germany) who have mild to moderate cognitive impairment. Eligibility criteria (patient participant): Patient inclusion criteria:
Patient exclusion criteria:
Eligibility criteria (caregiver participant): Family members who provided care for the fracture patients and met the following inclusion criteria were also invited to participate in the study. Caregiver inclusion criteria:
Caregiver exclusion criteria:
Group allocation: Patient participants were randomly assigned to the experimental or control group in a 1:1 ratio after the first assessment and before discharge from inpatient rehabilitation. Computer‐generated random allocation was done by an independent randomisation centre, and sealed envelopes were used. Diagnosis/cognitive status: Patient participants with an MMSE score of 17 to 26 points. Age: Patient participants aged 65 years and over Gender mix: Not specified Usual place of residence: Not specified Surgical management: Not specified Comorbidities: Not specified |
| Interventions |
Intervention arm: Usual care plus a multifactorial OF‐CARE intervention that centres on: physical activity promotion; an individually tailored, progressive exercise home programme; and care counselling for the participants and their participating caregivers (if existing). Home visit provided 2 to 6 weeks postdischarge (maximum 2 hours duration) developed by an exercise instructor (physiotherapist or sports scientist) and a lay instructor. The aim of this visit is: (a) to set at least 1 physical activity goal; (b) to specify a tailored exercise programme on strength, balance, and gait; and (c) to introduce and instruct a lay instructor. Activity goals are set using a card‐sorting exercise, providing a visual representation of activities to develop goals towards. Importance of physical activity and exercise were discussed with participants. Individually tailored training programme undertaken with balance and strength exercises, to meet the needs of participants. Lay instructors then visit twice‐weekly for 4 months (each visit maximum 2 hours) where the exercise programme is supervised. The exercise instructor supervises the lay instructor by a minimum of 5 telephone calls or email contacts and a further 2 home visits. During these, the exercise components are reviewed and adapted if needed and environmental assessment made. Exercise instructor also telephones each participant 5 times during the intervention period to feedback progress and address any issues raised. In addition, at least 1 of the following skills/interventions are delivered during each of the 3 home visits the exercise instructor performs: (1) addressing a minimum of 3 fall hazards and options for modification; (2) identifying situations in which the participant feels insecure when walking or experiences fear of falling and discussing coping strategies; (3) using walking aids safely; (4) possible self‐help strategies after a fall has occurred; (5) discussing or practising backward chaining as a strategy to get up independently from the floor; and (6) further physical activity promotion (e.g. resuming daily activities and routines, participation in community activities or local exercise classes). In addition, after the initial visit, a care counsellor is contacted. They are informed about the patient participant’s goals and unmet care needs. The care counsellor then intervenes during 1 initial home visit (maximum duration 2.5 hours) and up to 5 telephone calls throughout the intervention period. They work to facilitate the participant’s daily routines, pleasurable activities, participation, and adequate care needs. This is to the participant and (if existing) the principal caregiver. The caregiver receives a standardised problem‐solving intervention and information via a booklet on caregiver issues, falls prevention, memory aids for participants, skills to recognise and dealing with care recipient’s pain or depressive symptoms and recommended environmental adjustments, nutrition, and how to behave in the instance of a participant fall. Information is provided to caregivers on local supported and health promotion strategies. Control arm: Usual healthcare provision. All participants receive a face‐to‐face advice session (maximum duration 60 minutes) on recommended regular physical exercises and tips for fall prevention. These are summarised in an illustrated advice booklet. |
| Outcomes |
Time points: (‐T1) end of inpatient rehabilitation, only participant; (T1) pre‐intervention at participant’s home: week 2 to 6 postdischarge; (T2) postintervention at participant’s home: 4 months after T1; (T3) follow‐up at participant’s home: 3 months after T2 Outcomes (patient participant): Daily walking duration (24 hours) using a thigh‐worn inertial senior for 3 consecutive weekdays (activPAL3, PAL Technologies Ltd, Glasgow, UK); Short Physical Performance Battery (SPPB); Fear of Falling Questionnaire‐revised (FFQ‐R); Short Falls Efficacy Scale‐International (Short FES‐I); falls number (diary); Quality Of Life in Alzheimer’s Disease questionnaire (QOL‐AD); Montgomery‐Asberg Depression Rating Scale (MADRS); Barthel Index (BI); daily activity profile measured with activPAL3 including average daily number of steps, number of walking bouts, daily upright duration, daily number of sit‐to‐stand transfers; functional performance using an accelerometer (DynaPort Hybrid, McRoberts, The Hague, the Netherlands) to assess: sway area and sway path; angular velocity and fastest sit‐to‐stand; Western Ontario and McMaster Universities Osteoarthritis‐Scale Pain subset; Nuremberg Age Inventory (NAI:ZN‐G); modified German Social Support Questionnaire (F‐SozU Part B); economic evaluation questionnaire (health costs, care costs, and intervention costs); adverse events. Outcomes (caregiver participant): Centre for Epidemiological Studies ‐ Depression Scale (CES‐D), Sense of Competence Questionnaire (SCQ); Carer‐related Quality of Life questionnaire (CarerQoL); Giessen Subjective Complaints List (GSCL); Social Problem‐Solving Inventory ‐ revised/subscale (SPSI‐R:S); Leisure Time Satisfaction Measure (LTS); Revised Memory and Behaviour Problems Checklist/subscale frequency (RMBPC); economic evaluation questionnaire (health costs, care costs, and intervention costs); Time Burden Questionnaire ‐ including 3 dimensions of care ((1) body care; nutrition, mobility, (2) household help (e.g. housekeeping), (3) additional supervision). Adherence to the intervention was recorded for both patient participant and caregiver participant intervention provision. |
| Starting date | 27 July 2015 |
| Contact information | Dr Klaus Pfeiffer, Robert Bosch Hospital, Clinic for Geriatric Rehabilitation, Auerbachstr. 110, Stuttgart, 70376, Germany. Email: Klaus.Pfeiffer@rbk.de |
| Notes |
Proposed end date: January 2019 ISRCTN registration: ISRCTN69957256 Trial registration last updated: 2 May 2019 ‐ completed Recruitment process acknowledging cognitive impairment: Existing legal guardians or authorised representatives were involved in the information and consent process in any case, otherwise the closest family member if possible. |
Hammond 2017.
| Trial name or title | PERFECTED enhanced recovery (PERFECT‐ER) care versus standard acute care for patients admitted to acute settings with hip fracture identified as experiencing confusion: study protocol for a feasibility cluster randomised controlled trial. |
| Methods | A feasibility cluster‐randomised controlled trial comparing PERFECTED enhanced recovery (PERFECTER‐ER) vs standard care. |
| Participants |
Number: Will recruit 400 hip fracture patients identified as experiencing confusion (also suitable informants who will complete proxy measures). Eligibility criteria: Patient inclusion criteria:
Patient exclusion criteria:
Suitable informant inclusion criteria:
Suitable informant exclusion criteria: Individual not over 16 years of age. Group allocation: Cluster‐randomised across hospital wards in 10 NHS hospitals located in 5 different UK regions. Each hospital contributes 1 ward, and the unit of randomisation is the hospital site. An ad hoc programme will be written in SAS to carry out this procedure. 40 participants in 10 different sites. Diagnosis/cognitive status: The Abbreviated Mental Test Score (AMTS) and the 4A Test: screening instrument for cognitive impairment and delirium (4AT). Age: 60 years old and over Gender mix: Not specified Usual place of residence: Not specified Surgical management: Not specified Comorbidities: Not specified |
| Interventions |
Intervention arm: PERFECT‐ER pathway. As the current paper is reporting the protocol for a feasibility study, the intervention is understandably not yet in the public domain. Control arm: Control is treatment as usual. Local practices in each site will differ. We will collect relevant site profile data (please see “Site profile data” for details). |
| Outcomes |
Time points: 1 month +/‐ 5 days; 3 months +/‐ 5 days; 6 months +/‐ 5 days Outcomes: MMSE‐2 (participant); Dementia Quality of Life (DEMQOL) (participant and informant); EQ‐5D‐5L (participant and informant); howRwe (participant); howRthey (informant); Clinical Dementia Rating (CDR) (participant and informant); patient care profile (participant); timed up and go (participant); Bristol Activities of Daily Living Scale (informant); EQ‐5D‐5L carer self‐report (informant); client service receipt inventory (CSRI) (informant); number of days in institutional care (informant); participant place of residence (informant); Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (informant); length of hospital stay; discharge destination; mortality; hospital readmission; hospital service use; 4AT; Charlson Comorbidity Index (CCI). |
| Starting date | 1 November 2016 |
| Contact information | Professor Chris Fox ‐ Norwich Medical School, University of East Anglia, Norwich, NR4 7TJ. Email: chris.fox@uea.ac.uk |
| Notes |
Proposed end date: August 2018 ISRCTN registration: ISRCTN99336264 Trial registration last updated: 20 February 2018 ‐ completed Site inclusion criteria: Sites have an average monthly admission of at least 12 individuals who sustain proximal hip fracture requiring an operation and have a preoperative AMTS ≤ 8 (England) or a 4AT ≥ 1 (Scotland) in the last 12 available calendar months. Sites are able to provide the PERFECTED trial team with contextual ward‐level data (comprising BPT scores, number of falls, pressure ulcers, deaths, and safeguarding incidents) in the last 12 available calendar months. Sites that have participated in the 'PERFECTED WP2: Implementing optimised hospital care' research programme leading to the development and refinement of PERFECT‐ER will be excluded. |
ADLs: Activities of Daily Living
BPT: Best Practice Tariff
CTIMP: Clinical Trial of an Investigational Medicinal Product MMSE: Mini‐Mental State Examination
Differences between protocol and review
We did not identify any trials assessing the effectiveness of a rehabilitation or care model intended specifically for people with dementia following hip fracture in the search conducted for the first version of the review, therefore we amended the methods from the original protocol to include trials of enhanced rehabilitation and care models designed for all older people following hip fracture. We added determining the effectiveness of models of care including enhanced rehabilitation strategies that are designed for all older people, regardless of cognitive status, following hip fracture surgery compared to usual care, as a second objective.
We also broadened the criteria for types of participants to be included in the review. In addition to people with dementia diagnosed using validated diagnostic criteria, we also included trials reporting data on people with chronic cognitive impairment likely to be due to dementia, which we considered to be a better reflection of the way in which people might be selected for interventions in practice.
We clarified the terminology around rehabilitation and care models for the review. Since the aim of the review was to examine what can be drawn from the current literature to help devise an intervention specifically for people with dementia, we recognised that we needed to assess care models involving all multidisciplinary interventions along the patient's care pathway, not just conventionally interpreted rehabilitation from physiotherapists and occupational therapists. We amended the title of the review and the terminology in the review to reflect this.
We stated in the protocol that we would assess the certainity of the outcome related to the primary and first five secondary outcome measures using the GRADE approach. We amended this in the review to apply GRADE ratings to all outcome measures.
Given the limited number of eligible papers identified by the search strategy, it was not possible to construct a funnel plot to assess small‐sample‐size publication bias or to undertake sensitivity analyses for pooled data.
Further differences from protocol in second version of the review
Prior to undertaking the first update of the review, we revised the list of primary outcomes. We selected quality of life and functional performance as the primary outcomes of the review, whilst the previous primary outcome of cognitive function became a secondary outcome. We made this change in order to increase the focus on physical recovery, which is the principal objective of rehabilitative care after hip fracture.
We reviewed and updated the Background text based on more recent literature.
Our trials of interest test complex interventions, undertaken in different settings. Accordingly, we made a decision postprotocol to conduct all meta‐analyses using a random‐effects model when clinical homogeneity indicated that pooling of data was appropriate.
We originally defined endpoint categories as short term (surgery to three months postoperatively), medium term (three to 12 months postoperatively), and long term (more than 12 months postoperatively). For this update, we categorised 'short term' as surgery to four months because four months has become the short‐term assessment time point for hip fracture outcomes internationally (Sund 2011; Gjertsen 2016).
We did not report number needed to treat and absolute risk difference in the 'Summary of findings' tables. This was deemed appropriate since most of the results were of very low overall certainty and were consistent with both benefit and harm from the experimental intervention.
Contributions of authors
TS: Contributed to the literature search; reviewed the search results for eligibility; identified all included trials; independently performed data extraction; assessed risk of bias of the included trials; conducted data analysis; was involved in the writing and approval of the protocol and the final review; acts as guarantor.
AG: Contributed to the literature search; reviewed the search results for eligibility; identified all included trials; independently performed data extraction; assessed risk of bias of the included trials; provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
AS: Contributed to the assessment of risk of bias of the included trials; prepared the 'Summary of findings' tables; provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
OS: Provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
XG: Provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
JC: Provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
CF: Adjudicated data extraction and 'Risk of bias' assessment; provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
SL: Provided judgements on the interpretation of the results and conclusions drawn; was involved in the writing and approval of the protocol and the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This review will form part of a NIHR Programme Grant (Reference Number: DTC‐RP‐PG‐0311‐10004; Chief Investigator: Fox)
-
National Institute for Health Research, UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
-
National Institute for Health Research, Oxford Biomedical Research Centre, UK.
Dr Smith and Professor Lamb are supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Declarations of interest
Toby O Smith: none known
Anthony W Gilbert: none known
Ashwini Sreekanta: none known
Opinder Sahota: none known
Xavier L Griffin: none known
Jane L Cross: none known
Chris Fox: none known
Sarah E Lamb: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Freter 2017 {published data only}
- Freter S. Cochrane Hip Fracture and Dementia Data Question [personal communication]. Email to: TO Smith 25 September 2019.
- Freter S. Cochrane Hip Fracture and Dementia Data [personal communication]. Email to: Smith TO 17 January 2019.
- Freter S. Cochrane Hip Fracture and Dementia [personal communication]. Email to: TO Smith 15 November 2018.
- Freter S, Koller K, Dunbar M, MacKnight C, Rockwood K. Translating delirium prevention strategies for elderly adults with hip fracture into routine clinical care: a pragmatic clinical trial. Journal of the American Geriatrics Society 2017;65(3):567‐73. [DOI: 10.1111/jgs.14568] [DOI] [PubMed] [Google Scholar]
Huusko 2000 {published data only}
- Huusko TM, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Intensive geriatric rehabilitation of hip fracture patients. Acta Orthopaedica Scandinavica 2002;73:425‐31. [DOI] [PubMed] [Google Scholar]
- Huusko TM, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Randomised, clinically controlled trial of intensive geriatric rehabilitation in patients with hip fracture: subgroup analysis of patients with dementia. BMJ 2000;321(7269):1107‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcantonio 2001 {published data only}
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. Journal of the American Geriatric Society 2001;49(5):516‐22. [DOI] [PubMed] [Google Scholar]
Shyu 2012 {published data only}
- Shyu YI, Tsai WC, Chen MC, Liang J, Cheng HS, Wu CC, et al. Two‐year effects of an interdisciplinary intervention on recovery following hip fracture in older Taiwanese with cognitive impairment. International Journal of Geriatric Psychiatry 2012;27(5):529‐38. [DOI] [PubMed] [Google Scholar]
- Shyu YI, Tseng MY, Liang J, Tsai WC, Wu CC, Cheng HS. Interdisciplinary intervention decreases cognitive impairment for older Taiwanese with hip fracture: 2‐year follow‐up. International Journal of Geriatric Psychiatry 2013;28(12):1222‐31. [DOI] [PubMed] [Google Scholar]
- Shyu YU. Cochrane Hip Fracture and Dementia [personal communication]. Email to: TO Smith 17 October 2013.
Stenvall 2012 {published data only}
- Lundström M, Olofsson B, Stenvall M, Karlsson S, Nyberg L, Englund U, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clinical and Experimental Research 2007;19(3):178‐86. [DOI] [PubMed] [Google Scholar]
- Stenvall M. Cochrane Hip Fracture and Dementia [personal communication]. Email to: TO Smith 30 November 2013.
- Stenvall M, Berggren M, Lundström M, Gustafson Y, Olofsson B. A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia ‐ subgroup analyses of a randomized controlled trial. Archives of Gerontology and Geriatrics 2012;54(3):284‐9. [DOI] [PubMed] [Google Scholar]
Uy 2008 {published data only}
- Uy C, Kurrle SE, Cameron ID. Inpatient multidisciplinary rehabilitation after hip fracture for residents of nursing homes: a randomised trial. Australasian Journal on Ageing 2008;27(1):43‐4. [DOI] [PubMed] [Google Scholar]
Wyller 2012 {published and unpublished data}
- Watne LO, Torbergsen AC, Conroy S, Engedal K, Frihagen F, Hjorthaug GA, et al. The effect of a pre‐ and postoperative orthogeriatric service on cognitive function in patients with hip fracture: randomized controlled trial (Oslo Orthogeriatric Trial). BMC Medicine 2014;12:63. [DOI: 10.1186/1741-7015-12-63] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watne O. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 26 September 2019.
- Watne O. Cochrane Hip Fracture and Dementia [personal communication]. Email to: TO Smith 10 October 2019.
- Wyller TB, Watne LO, Torbergsen A, Engedal K, Frihagen F, Juliebø V, et al. The effect of a pre‐ and post‐operative orthogeriatric service on cognitive function in patients with hip fracture. The protocol of the Oslo Orthogeriatrics Trial. BMC Geriatrics 2012;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adunsky 2003a {published data only}
- Adunsky A, Lusky A, Arad M, Heruti RJ. A comparative study of rehabilitation outcomes of elderly hip fracture patients: the advantage of a comprehensive orthogeriatric approach. Journals of Gerontology: Series A Biological Sciences and Medical Sciences 2003;58A(6):542‐7. [DOI] [PubMed] [Google Scholar]
Arinzon 2010 {published data only}
- Arinzon Z, Shabat S, Peisakh A, Gepstein R, Berner YN. Gender differences influence the outcome of geriatric rehabilitation following hip fracture. Archives of Gerontology and Geriatrics 2010;50(1):86‐91. [DOI] [PubMed] [Google Scholar]
Berggren 2019 {published data only}
- Berggren M, Karlsson A, Lindelof N, Englund U, Olofsson B, Nordstrom P, et al. Effects of geriatric interdisciplinary home rehabilitation on complications and readmissions after hip fracture: a randomized controlled trial. Clinical Rehabilitation 2019;33(1):64‐73. [PUBMED: 30064264] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bongartz 2017 {published data only}
- Bongartz M, Kiss R, Ullrich P, Eckert T, Bauer J, Hauer K. Development of a home‐based training program for post‐ward geriatric rehabilitation patients with cognitive impairment: study protocol of a randomized‐controlled trial. BMC Geriatrics 2017;17(1):214. [DOI: 10.1186/s12877-017-0615-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 1993 {published data only}
- Cameron ID. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 10 April 2019.
- Cameron ID. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 15 November 2018.
- Cameron ID. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 18 January 2019.
- Cameron ID. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 21 March 2019.
- Cameron ID, Lyle DM, Quine S. Accelerated rehabilitation after proximal femoral fracture: a randomized controlled trial. Disability and Rehabilitation 1993;15(1):29‐34. [PUBMED: 8431589] [DOI] [PubMed] [Google Scholar]
- Cameron ID, Lyle DM, Quine S. Cost effectiveness of accelerated rehabilitation after proximal femoral fracture. Journal of Clinical Epidemiology 1994;47(11):1307‐13. [PUBMED: 7722567] [DOI] [PubMed] [Google Scholar]
Chong 2013 {published data only}
- Chong TW, Chan G, Feng L, Goh S, Hew A, Ng TP, et al. Integrated care pathway for hip fractures in a subacute rehabilitation setting. Annals, Academy of Medicine, Singapore 2013;42(11):579‐84. [PUBMED: 24356654] [PubMed] [Google Scholar]
Crotty 2003 {published data only}
- Crotty M, Whitehead C, Miller M, Gray S. Patient and caregiver outcomes 12 months after home‐based therapy for hip fracture: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2003;84(8):1237‐9. [PUBMED: 12917867] [DOI] [PubMed] [Google Scholar]
Crotty 2019 {published data only}
- Crotty M, Killington M, Liu E, Cameron ID, Kurrle S, Kaambwa B, et al. Should we provide outreach rehabilitation to very old people living in nursing care facilities after a hip fracture? A randomised controlled trial. Age and Ageing 2019;48(3):373‐80. [PUBMED: 30794284] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunliffe 2004 {published data only}
- Cunliffe AL, Gladman JR, Husbands SL, Miller P, Dewey ME, Harwood RH. Sooner and healthier: a randomised controlled trial and interview study of an early discharge rehabilitation service for older people. Age and Ageing 2004;33(3):246‐52. [DOI: 10.1093/ageing/afh076; PUBMED: 15082429] [DOI] [PubMed] [Google Scholar]
Deschodt 2011 {published data only}
- Deschodt M, Braes T, Broos P, Sermon A, Boonen S, Flamaing J, et al. Effect of an inpatient geriatric consultation team on functional outcome, mortality, institutionalization, and readmission rate in older adults with hip fracture: a controlled trial. Journal of the American Geriatric Society 2011;59(7):1299‐308. [DOI] [PubMed] [Google Scholar]
Espaulella 2000 {published data only}
- Espaulella J, Guyer H, Diaz‐Escriu F, Mellado‐Navas JA, Castells M, Pladevall M. Nutritional supplementation of elderly hip fracture patients. A randomized, double‐blind, placebo‐controlled trial. Age and Ageing 2000;29(5):425‐31. [DOI] [PubMed] [Google Scholar]
Flikweert 2014 {published data only}
- Flikweert ER, Izaks GJ, Knobben BA, Stevens M, Wendt K. The development of a comprehensive multidisciplinary care pathway for patients with a hip fracture: design and results of a clinical trial. BMC Musculoskeletal Disorders 2014;15(1):188. [PUBMED: 24885674] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauer 2017 {published data only}
- Hauer K. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 16 November 2018.
- Hauer K, Ullrich P, Dutzi I, Beurskens R, Kern S, Bauer J, et al. Effects of standardized home training in patients with cognitive impairment following geriatric rehabilitation: a randomized controlled pilot study. Gerontology 2017;63(6):495‐506. [DOI: 10.1159/000478263] [DOI] [PubMed] [Google Scholar]
Heruti 1999 {published data only}
- Heruti RJ, Lusky A, Barell V, Ohry A, Adunsky A. Cognitive status at admission: does it affect the rehabilitation outcome of elderly patients with hip fracture?. Archives of Physical Medicine and Rehabilitation 1999;80(4):432‐6. [DOI] [PubMed] [Google Scholar]
Horgan 2003 {published data only}
- Horgan NF, Cunningham CJ. Impact of cognitive impairment on hip fracture outcome in older people. International Journal of Therapy and Rehabilitation 2003;10(5):228‐32. [Google Scholar]
Jensen‐Dahm 2016 {published data only}
- Jensen‐Dahm C, Palm H, Gasse C, Dahl JB, Waldemar G. Postoperative treatment of pain after hip fracture in elderly patients with dementia. Dementia and Geriatric Cognitive Disorders 2016;41(3‐4):181‐91. [PUBMED: 27045590] [DOI] [PubMed] [Google Scholar]
Kalisvaart 2005 {published data only}
- Kalisvaart KJ, Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TC, Burger BJ, et al. Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study. Journal of the American Geriatric Society 2005;53(10):1658‐66. [DOI] [PubMed] [Google Scholar]
Karlsson 2016 {published data only}
- Karlsson A, Berggren M, Gustafson Y, Olofsson B, Lindelof N, Stenvall M. Effects of geriatric interdisciplinary home rehabilitation on walking ability and length of hospital stay after hip fracture: a randomized controlled trial. Journal of the American Medical Directors Association 2016;17(5):464.e9‐e15. [DOI: 10.1016/j.jamda.2016.02.001] [DOI] [PubMed] [Google Scholar]
Kennie 1988 {published data only}
- Kennie DC, Reid J, Richardson IR, Kiamari AA, Kelt C. Effectiveness of geriatric rehabilitative care after fractures of the proximal femur in elderly women: a randomised clinical trial. BMJ 1988;297(6656):1083‐6. [PUBMED: 3143436] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lima 2016 {published data only}
- Lima CA, Sherrington C, Guaraldo A, Moraes SA, Varanda RD, Melo JA, et al. Effectiveness of a physical exercise intervention program in improving functional mobility in older adults after hip fracture in later stage rehabilitation: protocol of a randomized clinical trial (REATIVE study). BMC Geriatrics 2016;16(1):198. [DOI: 10.1186/s12877-016-0370-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangione 2005 {published data only}
- Mangione KK. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 15 November 2018.
- Mangione KK, Craik RL, Tomlinson SS, Palombaro KM. Can elderly patients who have had a hip fracture perform moderate‐to high‐intensity exercise at home?. Physical Therapy 2005;85(8):727‐39. [PMID: 16048421] [PubMed] [Google Scholar]
Mangione 2010 {published data only}
- Mangione KK. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 15 November 2018.
- Mangione KK, Craik RL, Palombaro KM, Tomlinson SS, Hofmann MT. Home‐based leg‐strengthening exercise improves function 1 year after hip fracture: a randomized controlled study. Journal of the American Geriatrics Society 2010;58(10):1911‐7. [DOI: 10.1111/j.1532-5415.2010.03076x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martín‐Martín 2014 {published data only}
- Martín‐Martín LM, Valenza‐Demet G, Jiménez‐Moleón JJ, Cabrera‐Martos I, Revelles‐Moyano FJ, Valenza MC. Effect of occupational therapy on functional and emotional outcomes after hip fracture treatment: a randomized controlled trial. Clinical Rehabilitation 2014;28(6):541‐51. [DOI: 10.1177/0269215513511472; PUBMED: 24271264] [DOI] [PubMed] [Google Scholar]
McGilton 2009 {published data only}
- McGilton KS, Mahomed N, Davis AM, Flannery J, Calabrese S. Outcomes for older adults in an inpatient rehabilitation facility following hip fracture (HF) surgery. Archives of Gerontology and Geriatrics 2009;49(1):e23‐31. [DOI] [PubMed] [Google Scholar]
Morrison 2000 {published data only}
- Morrison RS, Siu AL. A comparison of pain and its treatment in advanced dementia and cognitively intact patients with hip fracture. Journal of Pain and Symptom Management 2000;19(4):240‐8. [DOI] [PubMed] [Google Scholar]
Moseley 2009 {published data only}
- Cameron ID. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 10 April 2019.
- Cameron ID. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 15 November 2018.
- Cameron ID. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 16 January 2019.
- Cameron ID. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 21 March 2019.
- Moseley AM, Sherrington C, Lord SR, Barraclough E, George RJ, Cameron ID. Mobility training after hip fracture: a randomised controlled trial. Age and Ageing 2009;38(1):74‐80. [DOI: 10.1093/ageing/afn217; PUBMED: 19017676] [DOI] [PubMed] [Google Scholar]
Naglie 2002 {published data only}
- Naglie G, Tansey C, Kirkland JL, Ogilvie‐Harris DJ, Detsky AS, Etchells E, et al. Interdisciplinary inpatient care for elderly people with hip fracture: a randomized controlled trial. Canadian Medical Association Journal 2002;167(1):25‐32. [PMC free article] [PubMed] [Google Scholar]
Oldmeadow 2006 {published data only}
- Oldmeadow LB, Edwards ER, Kimmel LA, Kipen E, Robertson VJ, Bailey MJ. No rest for the wounded: early ambulation after hip surgery accelerates recovery. Australian and New Zealand Journal of Surgery 2006;76(7):607‐11. [DOI: 10.1111/j.1445-2197.2006.03786.x; PUBMED: 16813627] [DOI] [PubMed] [Google Scholar]
O’Halloran 2016 {published data only}
- O’Halloran PD, Shields N, Blackstock F, Wintle E, Taylor NF. Motivational interviewing increases physical activity and self‐efficacy in people living in the community after hip fracture: a randomized controlled trial. Clinical Rehabilitation 2016;30(11):1108‐19. [DOI] [PubMed] [Google Scholar]
Penrod 2004 {published data only}
- Penrod JD, Boockvar KS, Litke A, Magaziner J, Hannan EL, Halm EA, et al. Physical therapy and mobility 2 and 6 months after hip fracture. Journal of the American Geriatric Society 2004;52(7):1114‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitkala 2006 {published data only}
- Pitkälä KH, Laurila JV, Strandberg TE, Tilvis RS. Multicomponent geriatric intervention for elderly inpatients with delirium: a randomized, controlled trial. Journal of Gerentology: Medical Sciences 2006;61A(2):176‐81. [DOI] [PubMed] [Google Scholar]
Reguant 2019 {published data only}
- Reguant F, Arnau A, Lorente JV, Maestro L, Bosch J. Efficacy of a multidisciplinary approach on postoperative morbidity and mortality of elderly patients with hip fracture. Journal of Clinical Anesthesia 2019;53:11‐9. [PUBMED: 30286380] [DOI] [PubMed] [Google Scholar]
Rolland 2004 {published data only}
- Rolland Y, Pillard F, Lauwers‐Cances V, Busquère F, Vellas B, Lafont C. Rehabilitation outcome of elderly patients with hip fracture and cognitive impairment. Disability and Rehabilitation 2004;26(7):425‐31. [DOI] [PubMed] [Google Scholar]
Schwenk 2014 {published data only}
- Hauer K. Cochrane Hip Fracture and Dementia Review [personal correspondence]. Email to: TO Smith 15 November 2018.
- Schwenk M, Dutzi I, Englert S, Micol W, Najafi B, Mohler J, et al. An intensive exercise program improves motor performances in patients with dementia: translational model of geriatric rehabilitation. Journal of Alzheimer's Disease 2014;39:487‐98. [DOI: 10.3233/JAD-130470] [DOI] [PubMed] [Google Scholar]
Seitz 2011a {published data only}
- Seitz DP, Gill SS, Gruneir A, Austin PC, Anderson G, Reimer CL, et al. Effects of cholinesterase inhibitors on postoperative outcomes of older adults with dementia undergoing hip fracture surgery. American Journal of Geriatric Psychiatry 2011;19(9):803‐13. [DOI] [PubMed] [Google Scholar]
Sherrington 1997 {published data only}
- Sherrington C, Lord SR. Home exercise to improve strength and walking velocity after hip fracture: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 1997;78(2):208‐12. [PUBMED: 9041904] [DOI] [PubMed] [Google Scholar]
Stenvall 2007 {published data only}
- Stenvall M, Olofsson B, Lundström M, Englund U, Borssén B, Svensson O, et al. A multidisciplinary, multifactorial intervention program reduces postoperative falls and injuries after femoral neck fracture. Osteoporosis International 2007;18(2):167‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strömberg 1999 {published data only}
- Strömberg L. Cochrane Hip Fracture and Dementia [personal correspondence]. Email to: TO Smith 15 October 2013.
- Strömberg L, Ohlén G, Nordin C, Lindgren U, Svensson O. Postoperative mental impairment in hip fracture patients. A randomized study of reorientation measures in 223 patients. Acta Orthopaedica Scandinavica 1999;70(3):250‐5. [DOI] [PubMed] [Google Scholar]
Vidan 2005 {published data only}
- Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. Journal of the American Geriatric Society 2005;53(9):1476‐82. [DOI] [PubMed] [Google Scholar]
Williams 2017 {published data only}
- Williams NH, Roberts JL, Din NU, Charles JM, Totton N, Williams M, et al. Developing a multidisciplinary rehabilitation package following hip fracture and testing in a randomised feasibility study: Fracture in the Elderly Multidisciplinary Rehabilitation (FEMuR). Health Technology Assessment 2017;21(44):1. [DOI: 10.3310/hta21440; PUBMED: 28836493] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Dautel 2019 {published data only}
- Dautel A, Eckert T, Gross M, Hauer K, Schaufele M, Lacroix A, et al. Multifactorial intervention for hip and pelvic fracture patients with mild to moderate cognitive impairment: study protocol of a dual‐centre randomised controlled trial (OF‐CARE). BMC Geriatrics 2019;19(1):125. [DOI: 10.1186/s12877-019-1133-z; PUBMED: 31039754] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammond 2017 {published data only}
- Hammond SP, Cross JL, Shepstone L, Backhouse T, Henderson C, Poland F, et al. PERFECTED enhanced recovery (PERFECT‐ER) care versus standard acute care for patients admitted to acute settings with hip fracture identified as experiencing confusion: study protocol for a feasibility cluster randomized controlled trial. Trials 2017;18(1):583. [DOI: 10.1186/s13063-017-2303-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adams 2013
- Adams AL, Shi J, Takayanagi M, Dell RM, Funahashi TT, Jacobsen SJ. Ten‐year hip fracture incidence rate trends in a large California population, 1997‐2006. Osteoporosis International 2013;24(1):373‐6. [PUBMED: 22349963] [DOI] [PubMed] [Google Scholar]
Adunsky 2003b
- Adunsky A, Levy R, Heim M, Mizrahi E, Arad M. The unfavorable nature of preoperative delirium in elderly hip fractured patients. Archives of Gerontology and Geriatrics 2003;36(1):67‐74. [DOI: 10.1016/S0167-4943(02)00058-4] [DOI] [PubMed] [Google Scholar]
Al‐Ani 2010
- Al‐Ani AN, Flodin L, Söderqvist A, Ackermann P, Samnegård E, Dalén N, et al. Does rehabilitation matter in patients with femoral neck fracture and cognitive impairment? A prospective study of 246 patients. Archives of Physical Medicine and Rehabilitation 2010;91(1):51‐7. [DOI: 10.1016/j.apmr.2009.09.005] [DOI] [PubMed] [Google Scholar]
Allen 2012
- Allen J, Koziak A, Buddingh S, Liang J, Buckingham J, Beaupre LA. Rehabilitation in patients with dementia following hip fracture: a systematic review. Physiotherapy Canada 2012;64(2):190‐201. [DOI: 10.3138/ptc.2011-06BH; PUBMED: 23449813] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alzheimer's Disease International 2020
- Alzheimer's Disease International. Dementia statistics. www.alz.co.uk/research/statistics (accessed 15 January 2020).
Alzheimer's Research UK 2018
- Alzheimer's Research UK Dementia Statistics Hub. Hospital. www.dementiastatistics.org/statistics/hospitals/ (accessed 10 June 2019).
Alzheimer's Society 2014
- Alzheimer's Society. Dementia UK report. www.alzheimers.org.uk/about‐us/policy‐and‐influencing/dementia‐uk‐report (accessed 10 June 2019).
American Psychiatric Association 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
Blessed 1968
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile changes in the cerebral grey matter of elderly subjects. British Journal of Psychiatry 1968;114(512):797‐811. [PUBMED: 5662937] [DOI] [PubMed] [Google Scholar]
Brandt 1991
- Brandt J. The Hopkins Verbal Learning Test: development of a new verbal memory test with six equivalent forms. Clinical Neuropsychologist 1991;5(2):125‐42. [DOI: 10.1080/13854049108403297] [DOI] [Google Scholar]
Bucks 1996
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia development of the Bristol Activities of Daily Living Scale. Age and Ageing 1996;25(2):113‐20. [PUBMED: 8670538] [DOI] [PubMed] [Google Scholar]
Cameron 2000
- Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al. Geriatric rehabilitation following fractures in older people: a systematic review. Health Technology Assessment 2000;4(2):i‐iv: 1‐111. [PUBMED: 10702905] [PubMed] [Google Scholar]
Charlson 1987
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Disease 1987;40(5):373‐83. [PUBMED: 3558716] [DOI] [PubMed] [Google Scholar]
Chartered Society of Physiotherapy 2018
- Chartered Society of Physiotherapy. Hip fracture rehabilitation standards. www.csp.org.uk/publications/hip‐fracture‐rehabilitation‐physiotherapy‐practice (accessed 24 April 2019).
Chu 2016
- Chu CH, Paquin K, Puts M, McGilton KS, Babineau J, Wyk PM. Community‐based hip fracture rehabilitation interventions for older adults with cognitive impairment: a systematic review. JMIR Rehabilitation and Assistive Technologies 2016;3(1):e3. [PUBMED: 28582255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 1986
- Cohen‐Mansfield J. Agitated behaviors in the elderly. II. Preliminary results in the cognitively deteriorated. Journal of the American Geriatric Society 1986;34(10):722‐7. [PUBMED: 3760436] [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings J, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308‐14. [PUBMED: 7991117] [DOI] [PubMed] [Google Scholar]
Dawson 1996
- Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. Journal of Bone and Joint Surgery ‐ British Edition 1996;78(2):185‐90. [PUBMED: 8666621] [PubMed] [Google Scholar]
Drummond 2005
- Drummond M, Sculpher MJ, Torrance GW, O'Brien B, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press, 2005. [Google Scholar]
Dy 2012
- Dy CJ, Dossous PM, Ton QV, Hollenberg JP, Lorich DG, Lane JM. The medical orthopaedic trauma service: an innovative multidisciplinary team model that decreases in‐hospital complications in patients with hip fractures. Journal of Orthopaedic Trauma 2012;26(6):379‐83. [PUBMED: 21885997] [DOI] [PubMed] [Google Scholar]
EuroQol Group 1990
- EuroQol Group. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16(3):199‐208. [PUBMED: 10109801] [DOI] [PubMed] [Google Scholar]
Feeny 2002
- Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, et al. Multiattribute and single‐attribute utility functions for the health utilities index mark 3 system. Medical Care 2002;40(2):113‐28. [PUBMED: 11802084] [DOI] [PubMed] [Google Scholar]
Feldt 1998
- Feldt KS, Ryden MB, Miles S. Treatment of pain in cognitively impaired compared with cognitively intact older patients with hip‐fracture. Journal of the American Geriatric Society 1998;46(9):1079‐85. [PUBMED: 9736099] [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein M, Folstein S, McHugh P. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189–98. [PUBMED: 1202204] [DOI] [PubMed] [Google Scholar]
Gill 2017
- Gill N, Hammond S, Cross J, Smith T, Lambert N, Fox C. Optimising care for patients with cognitive impairment and dementia following hip fracture. Zeitschrift fur Gerontologie und Geriatrie 2017;50 (Suppl 2):39‐43. [PUBMED: 28364260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gjertsen 2016
- Gjertsen JE, Baste V, Fevang JM, Furnes O, Engesaeter LB. Quality of life following hip fractures: results from the Norwegian hip fracture register. BMC Musculoskeletal Disorders 2016;17:265. [PUBMED: 27387741] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gladman 1993
- Gladman JR, Lincoln NB, Adams SA. Use of the extended ADL scale with stroke patients. Age and Ageing 1993;22(6):419‐24. [PUBMED: 8310887] [DOI] [PubMed] [Google Scholar]
Gruber‐Baldini 2003
- Gruber‐Baldini AL, Zimmerman S, Morrison RS, Grattan LM, Hebel JR, Dolan MM, et al. Cognitive impairment in hip fracture patients: timing of detection and longitudinal follow‐up. Journal of the American Geriatric Society 2003;51(9):1227‐36. [PUBMED: 12919234] [DOI] [PubMed] [Google Scholar]
Guralnik 2000
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. Journal of Gerontology Series A: Biological Sciences and Medical Sciences 2000;55(4):M221‐31. [PUBMED: 10811152] [DOI] [PubMed] [Google Scholar]
Handoll 2009
- Handoll HH, Cameron ID, Mak JC, Finnegan TP. Multidisciplinary rehabilitation for older people with hip fractures. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007125.pub2] [DOI] [PubMed] [Google Scholar]
Hashmi 2004
- Hashmi MA, Tellisi N, Rigby AS, Wahab KH. The value of a prognostic scoring system in the rehabilitation of elderly patients with proximal femoral fractures. International Journal of Clinical Practice 2004;58(1):2‐5. [PUBMED: 14994962] [DOI] [PubMed] [Google Scholar]
Haywood 2014
- Haywood KL, Griffin XL, Achten J, Costa ML. Developing a core outcome set for hip fracture trials. Bone and Joint Journal 2014;96‐B(8):1016‐23. [PUBMED: 25086115] [DOI] [PubMed] [Google Scholar]
Hebert‐Davies 2012
- Hebert‐Davies J, Laflamme GY, Rouleau D, HEALTH and FAITH investigators. Bias towards dementia: are hip fracture trials excluding too many patients? A systematic review. Injury 2012;43(12):1978‐84. [DOI: 10.1016/j.injury.2012.08.061] [DOI] [PubMed] [Google Scholar]
Henderson 2007
- Henderson C, Malley J, Knapp M. Maintaining Good Health for Older People With Dementia Who Experience Fractured Neck of Femur. London: National Audit Office: Personal Social Services Research Unit, London School of Economics and Political Science, 2007. [Google Scholar]
Hershkovitz 2010
- Hershkovitz A, Polatov I, Beloosesky Y, Brill S. Factors affecting mortality of frail hip‐fractured elderly patients. Archives of Gerontology and Geriatrics 2010;51(2):113‐6. [PUBMED: 19819572] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hodkinson 1972
- Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age and Ageing 1972;1(4):233‐8. [PUBMED: 4669880] [DOI] [PubMed] [Google Scholar]
Hunt 2009
- Hunt GR, Crealey G, Murthy BVS, Hall GM, Constantine P, O'Brien S, et al. The consequences of early discharge after hip arthroplasty for patient outcomes and healthcare costs: comparison of three centres with differing durations of stay. Clinical Rehabilitation 2009;23(12):1067‐77. [PUBMED: 19864466] [DOI] [PubMed] [Google Scholar]
Inouye 1990
- Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine 1990;113(12):941‐8. [PUBMED: 2240918] [DOI] [PubMed] [Google Scholar]
Jameson 2012
- Jameson SS, Khan SK, Baker P, James P, Gray A, Reed MR, et al. A national analysis of complications following hemiarthroplasty for hip fracture in older patients. QJM 2012;105(5):55‐60. [PUBMED: 22294648] [DOI] [PubMed] [Google Scholar]
Jorm 1989
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio‐demographic correlates, reliability, validity and some norms. Psychological Medicine 1989;19(4):1015‐22. [PUBMED: 2594878] [DOI] [PubMed] [Google Scholar]
Jorm 1994
- Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross‐validation. Psychological Medicine 1994;24(1):145‐53. [PUBMED: 8208879] [DOI] [PubMed] [Google Scholar]
Kammerlander 2010
- Kammerlander C, Roth T, Friedman SM, Suhm N, Luger TJ, Kammerlander‐Knauer U, et al. Ortho‐geriatric service – a literature review comparing different models. Osteoporosis International 2010;21(Suppl 4):S637‐46. [PUBMED: 21058004] [DOI] [PubMed] [Google Scholar]
Katz 1963
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. Journal of the American Medical Association 1963;185:914‐9. [PUBMED: 14044222] [DOI] [PubMed] [Google Scholar]
Knapp 2007
- Knapp M, Privette A. Dementia UK Alzheimer’s Society. London: Alzheimer’s Society, 2007. [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2(1):81. [PUBMED: 24059250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lenze 2007
- Lenze EJ, Skidmore ER, Dew MA, Butters MA, Rogers JC, Begley A, et al. Does depression, apathy or cognitive impairment reduce the benefit of inpatient rehabilitation facilities for elderly hip fracture patients?. General Hospital Psychiatry 2007;29(2):141‐6. [PUBMED: 17336663] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewiecki 2018
- Lewiecki EM, Wright NC, Curtis JR, Siris E, Gagel RF, Saag KG, et al. Hip fracture trends in the United States, 2002 to 2015. Osteoporosis International 2018;29(3):717‐22. [PUBMED: 29282482] [DOI] [PubMed] [Google Scholar]
Lieberman 2006
- Lieberman D, Friger M, Lieberman D. Inpatient rehabilitation outcome after hip fracture surgery in elderly patients: a prospective cohort study of 956 patients. Archives of Physical Medicine and Rehabilitation 2006;87(2):167‐71. [PUBMED: 16442967] [DOI] [PubMed] [Google Scholar]
Liu 2018
- Liu Y, Wang Z, Xiao W. Risk factors for mortality in elderly patients with hip fractures: a meta‐analysis of 18 studies. Aging Clinical and Experimental Research 2018;30(4):323‐30. [PUBMED: 28660596] [DOI] [PubMed] [Google Scholar]
Magaziner 1990
- Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. Journal of Gerontology 1990;45(3):M101‐7. [PUBMED: 2335719] [DOI] [PubMed] [Google Scholar]
Magaziner 2002
- Magaziner J, Hawkes W, Hebel JR, Zimmerman SI, Fox KM, Dolan M, et al. Recovery from hip fracture in eight areas of function. Journal of Gerontology Series A: Biological Sciences and Medical Sciences 2002;55(9):M498‐507. [PUBMED: 10995047] [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney F, Barthel D. Functional evaluation: the Barthel Index. Maryland Medical Journal 1965;14:61–5. [PUBMED: 14258950] [PubMed] [Google Scholar]
Mathuranath 2005
- Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology 2000;55(11):1613–20. [PUBMED: 11113213] [DOI] [PubMed] [Google Scholar]
Mattisson 2018
- Mattisson L, Bojan A, Enocson A. Epidemiology, treatment and mortality of trochanteric and subtrochanteric hip fractures: data from the Swedish fracture register. BMC Musculoskeletal Disorders 2018;19(1):369. [PUBMED: 30314495] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGilton 2012
- McGilton KS, Davis A, Mahomed N, Flannery J, Jaglal S, Cott C, et al. An inpatient rehabilitation model of care targeting patients with cognitive impairment. BMC Geriatrics 2012;12:21. [PUBMED: 22631877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menzies 2010
- Menzies IB, Mendelson DA, Kates SL, Friedman SM. Prevention and clinical management of hip fractures in patients with dementia. Geriatric Orthopaedic Surgery and Rehabilitation 2010;1(2):63‐72. [PUBMED: 23569664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morri 2018
- Morri M, Forni C, Marchioni M, Bonetti E, Marseglia F, Cotti A. Which factors are independent predictors of early recovery of mobility in the older adults' population after hip fracture? A cohort prognostic study. Archives of Orthopaedic and Trauma Surgery 2018;138(1):35‐41. [PUBMED: 28956152] [DOI] [PubMed] [Google Scholar]
Morrison 1998
- Morrison RS, Ahronheim JC, Morrison GR, Darling E, Baskin SA, Morris J, et al. Pain and discomfort associated with common hospital procedures and experiences. Journal of Pain and Symptom Management 1998;15(2):91‐101. [PubMed] [Google Scholar]
Nasreddine 2005
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment (MoCA): a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 2005;53(4):695‐9. [DOI] [PubMed] [Google Scholar]
NICE 2017
- NICE. Hip fracture: the management of hip fracture in adults. www.nice.org.uk/guidance/cg124 (accessed 24 April 2019).
Nouri 1987
- Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clinical Rehabilitation 1987;1:301‐5. [Google Scholar]
Parker 2010
- Parker MJ, Handoll HH. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD000093.pub5] [DOI] [PubMed] [Google Scholar]
Pfeiffer 1975
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly persons. Journal of the American Geriatrics Society 1975;23(10):433‐41. [PUBMED: 1159263] [DOI] [PubMed] [Google Scholar]
Resnick 2016
- Resnick B, Beaupre L, McGilton KS, Galik E, Liu W, Neuman MD, et al. Rehabilitation interventions for older individuals with cognitive impairment post‐hip fracture: a systematic review. Journal of the American Medical Directors Association 2016;17(3):200‐5. [PUBMED: 26612482] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosen 1984
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. American Journal of Psychiatry 1984;141(11):1356‐64. [PUBMED: 6496779] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ’Summary of findings’ tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Seaby 1989
- Seaby LTG, Torrance G. Reliability of a physiotherapy functional assessment in a rehabilitation setting. Physiotherapy Canada 1989;41(5):264‐71. [Google Scholar]
Seitz 2011b
- Seitz DP, Adunuri N, Gill SS, Rochon PA. Prevalence of dementia and cognitive impairment among older adults with hip fractures. Journal of the American Medical Directors Association 2011;12(8):556‐64. [PUBMED: 21450227] [DOI] [PubMed] [Google Scholar]
Seitz 2014
- Seitz DP, Gill SS, Gruneir A, Austin PC, Anderson GM, Bell CM, et al. Effects of dementia on postoperative outcomes of older adults with hip fractures: a population‐based study. Journal of the American Medical Directors Association 2014;15(5):334‐41. [PUBMED: 24524851] [DOI] [PubMed] [Google Scholar]
Smith 2005
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health‐related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technology Assessment 2005;9(10):1‐93; iii‐iv. [PUBMED: 15774233] [DOI] [PubMed] [Google Scholar]
Sonn 1996
- Sonn U. Longitudinal studies of dependence in daily life activities among elderly persons. Scandinavian Journal of Rehabilitation Medicine. Supplement 1996;34:1‐35. [PUBMED: 8701230] [PubMed] [Google Scholar]
Steiner 1997
- Steiner JF, Kramer AM, Eilertsen TB, Kowalsky JC. Development and validation of a clinical prediction rule for prolonged nursing home residence after hip fracture. Journal of the American Geriatric Society 1997;45(12):1510‐4. [PUBMED: 9400563] [DOI] [PubMed] [Google Scholar]
Sund 2011
- Sund R, Juntunen M, Lüthje P, Huusko T, Häkkinen U. Monitoring the performance of hip fracture treatment in Finland. Annals of Medicine 2011;43 Suppl 1:S39‐46. [PUBMED: 21639716] [DOI] [PubMed] [Google Scholar]
Söderqvist 2007
- Söderqvist A, Ponzer S, Tidermark J. Cognitive function and pressure ulcers in hip fracture patients. Scandinavian Journal of Caring Sciences 2007;21(1):79‐83. [PUBMED: 17428218] [DOI] [PubMed] [Google Scholar]
Tang 2017
- Tang VL, Sudore R, Cenzer IS, Boscardin WJ, Smith A, Ritchie C, et al. Rates of recovery to pre‐fracture function in older persons with hip fracture: an observational study. Journal of General Internal Medicine 2017;32(2):153‐8. [PUBMED: 27605004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trigg 2007
- Trigg R, Skevington SM, Jones RW. How can we best assess the quality of life of people with dementia? The Bath Assessment of Subjective Quality of Life in Dementia (BASQID). The Gerontologist 2007;47(6):789‐97. [PUBMED: 18192632] [DOI] [PubMed] [Google Scholar]
Uzoigwe 2012
- Uzoigwe CE, Burnand HG, Cheesman CL, Aghedo DO, Faizi M, Middleton RG. Early and ultra‐early surgery in hip fracture patients improves survival. Injury 2013;44(6):726‐9. [PUBMED: 23010072] [DOI] [PubMed] [Google Scholar]
Warden 2003
- Warden V, Hurley AC, Volicer V. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. Journal of the American Medical Directors Association 2003;4(1):9‐15. [PUBMED: 12807591] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PUBMED: 1593914] [PubMed] [Google Scholar]
Ware 1996
- Ware JE, Kosinski M, Keller SD. A 12‐item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34:220‐33. [PUBMED: 8628042] [DOI] [PubMed] [Google Scholar]
World Health Organization 2015
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 10th Revision. apps.who.int/classifications/icd10/browse/2015/en (accessed 31 May 2015).
References to other published versions of this review
Smith 2013
- Smith TO, Hameed YA, Henderson C, Cross JL, Sahota O, Fox C. Effectiveness of post‐operative management strategies for adults with dementia following hip fracture surgery. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010569] [DOI] [PubMed] [Google Scholar]


