Figure 1.
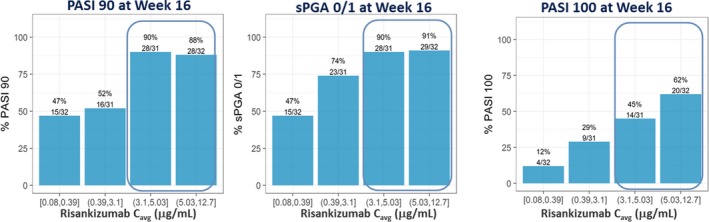
Relationships between risankizumab average plasma concentration (C avg) and observed PASI90, PASI100, and sPGA0/1 responses at week 16 in the phase II trial. Note: C avg represents average concentration from week 0 to 16. Median of C avg (μg/mL): quartile 1 = 0.24 μg/mL, quartile 2 = 1.97 μg/mL, quartile 3 = 3.97 μg/mL, and quartile 4 = 7.88 μg/mL. PASI, Psoriasis Area and Severity Index; sPGA, static Physician's Global Assessment. [Colour figure can be viewed at http://wileyonlinelibrary.com]
