Figure 4.
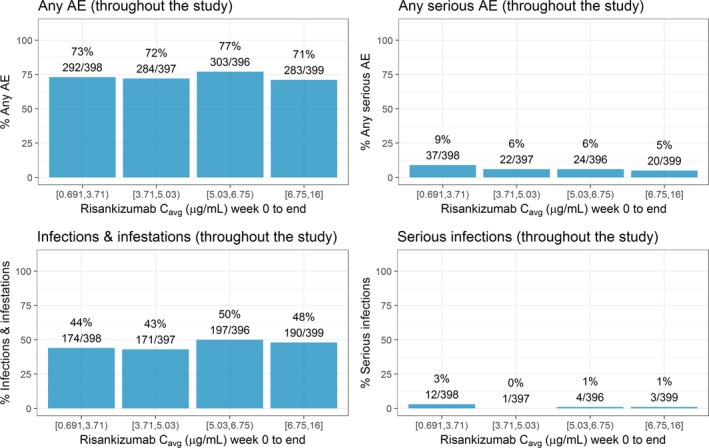
Exposure–response relationships between risankizumab plasma C avg and safety events of interest over the first 52 weeks treatment period. Note: Week 0 to end represents time period week 0–44 in IMMvent trial and week 0–52 in ultIMMa‐1, ultIMMa‐2, and IMMhance trials. C avg represents average concentration from week 0 to end. Median of C avg (μg/mL): quartile 1 = 2.98 μg/mL, quartile 2 = 4.25 μg/mL, quartile 3 = 5.87 μg/mL, quartile 4 = 7.93 μg/mL. AE, adverse event; C avg, average plasma concentration. [Colour figure can be viewed at http://wileyonlinelibrary.com]
