Figure 2.
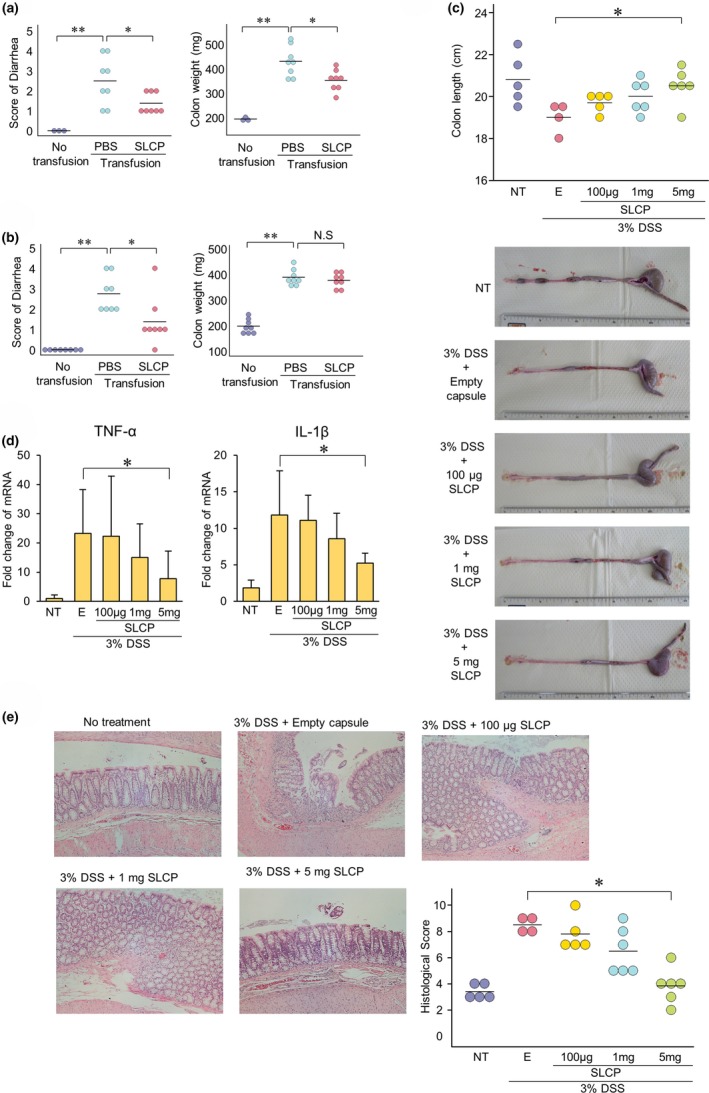
Long‐chain polyphosphate exerted treatment effects against inflammation both in vitro and in vivo. (a) The diarrhea score and colon weight of interleukin (IL)‐10 knockout (KO) transfusion mice recovered with transanal long‐chain polyphosphate treatment in comparison with the phosphate‐buffered saline (PBS) group (n = 6‐8). (b) The diarrhea score, but not the colon weight, of IL‐10 KO transfusion mice recovered with oral long‐chain polyphosphate treatment in comparison with the PBS group (n = 8). (c) To avoid exposure to acidic conditions and shortening of long‐chain polyphosphate, the polyphosphate was enclosed in gelatin capsules, and then enteric coating was performed. In the DSS‐induced colitis rat model, the colon length significantly recovered with the oral administration of enteric‐coated capsules containing long‐chain polyphosphate in the 5 mg/body polyphosphate group (n = 5). (d) The reverse transcription polymerase chain reaction revealed that in the DSS‐induced colitis rat model, the expression levels of TNF‐α and IL‐1β were significantly reduced by the administration of enteric‐coated polyphosphate capsules in the 5 mg/body polyphosphate group (n = 5). (e) In the DSS‐induced colitis rat model, the histopathological score recovered with the oral administration of enteric‐coated capsules containing long‐chain polyphosphate (n = 5). *P < 0.05, **P < 0.01 by Student's t‐test. The error bars show the standard deviation. DSS, dextran sodium sulfate; E, empty capsule; IL, interleukin; KO, knockout; LCP, long‐chain polyphosphate; NT, no treatment; PBS, phosphate‐buffered saline; TNF‐α, tumor‐necrosis factor alpha; Transfusion, IL‐10 KO‐derived T‐lymphocyte transfusion.
