Figure 6.
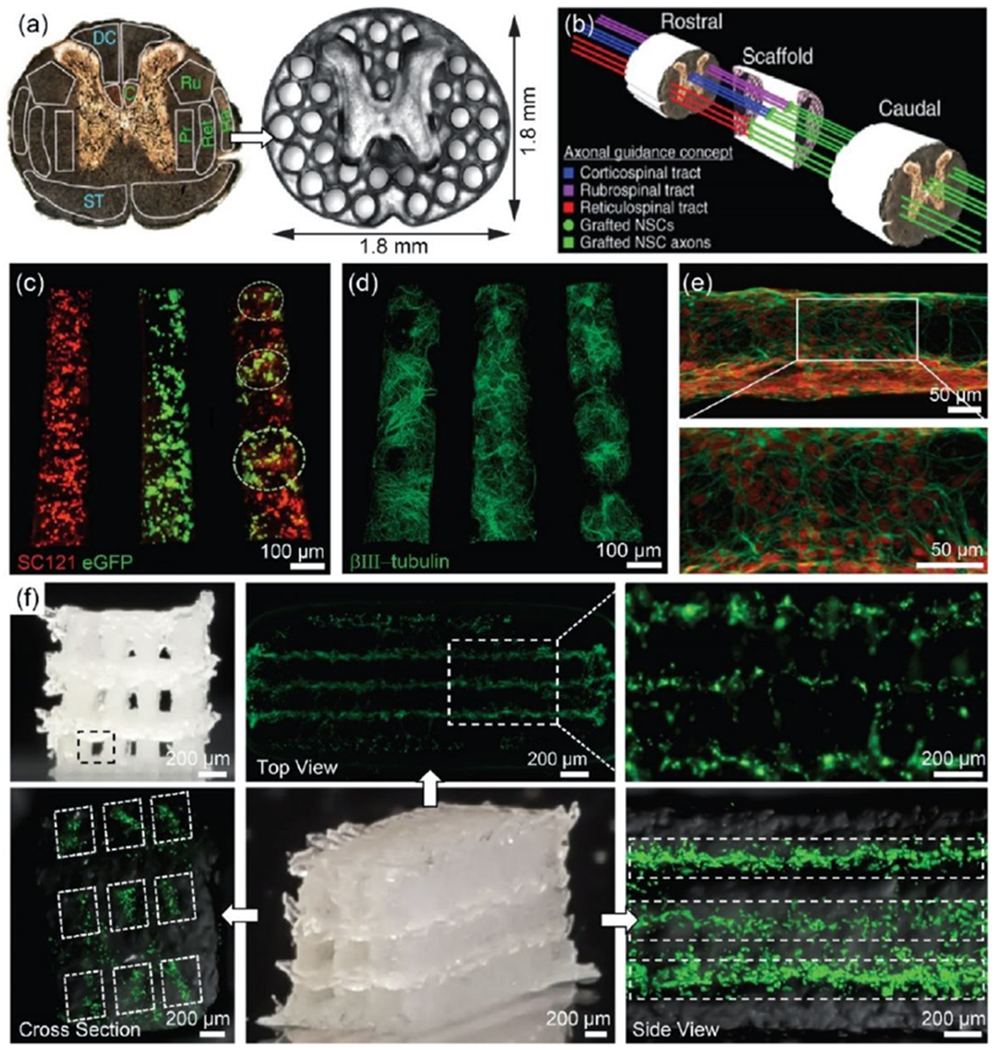
(a) PEGDA/GelMa hydrogel-based spinal cord scaffolds containing 200 μm–diameter multichannels, providing linear alignment of white matter with host axonal tracts in the spinal cord. Grey matter area was printed as a solid. (b) A schematic illustration of the axonal guidance using a 3D printed scaffold. Channels in the scaffold provide linear guidance of rostral-caudal planes, so that grafted cells and host cells can be aligned. Later, host axons in cortico-, rubro-, or reticulo-spinal tracts regenerate into the scaffold and form synaptic connections with grafted neurons inside the channels. The grafted neurons then extend axons, generating the functional connection across the lesion site. Reproduced with permission.[34] Copyright 2019, Nature Publishing Group. (c-e) 3D bioprinted sNPCS and OPCs in a 3D printed scaffold with ~150 μm-diameter channels. (c) Precise spatial distribution of neural cell types in specific channels after 1 day of culture: sNPCs (left), OPCs (middle), and sNPCs and OPCs (right). This allows for the recapitulation of spinal cord architecture with multiple cell types. (d) sNPCs printed in a scaffold after 4 days of culture. (e) Higher resolution image of 3D printed sNPCs in a channel. The sNPCs rapidly differentiated into neurons and extended axons propagating in the designed 3D space. (f) Neurocompatible 3D printed alginate-based scaffolds. The scaffold contained 3 × 3 channels, ~150 μm-diameter channels, and ~1.5 mm × 5 mm sized scaffold. Fluorescence images indicated that bioprinted sNPCs in a 3D printed scaffold were alive 3 days after printing in all three layers. Reproduced with permission.[15] Copyright 2018, Wiley-VCH.
