Abstract
Background
The mechanisms that govern the egress of mature thymocytes from the human thymus to the periphery remain understudied yet are of utmost importance to the field of basic immunology, as well as T-cell reconstitution in various immunodeficiencies. We examined the expression and function of sphingosine-1-phosphate (S1P) receptors in human thymocyte egress.
Objectives
We aimed to determine whether S1P receptors (S1P-Rs) play a role in mature human thymocyte egress and to identify the thymocyte population or populations that express S1P-Rs and respond to S1P by migrating across a concentration gradient.
Methods
Human thymocytes were exposed to S1P in Transwell plate migration assays coupled to flow cytometry to evaluate the response to S1P of thymocytes at different stages of maturation. Constitutive S1P-R expression was quantified by means of real-time PCR in sorted thymocyte subsets and flow cytometry. S1P-R1 and Kruppel-like factor 2 expression were monitored after S1P exposure by using flow cytometry and quantitative PCR.
Results
S1P-R1 was the prevalent S1P receptor on mature human thymocytes (CD3hiCD27+CD69−), the population that also demonstrated the greatest response to S1P in migration assays. Pretreatment with FTY720, an S1P-R1 nonselective modulator significantly reduced migration and suggested a role for S1P-R2 in retaining thymocytes in the tissue. Lastly, surface S1P-R1 expression, as well S1PR1 and Kruppel-like factor 2 (KLF2) transcripts, were significantly decreased in mature thymocytes on exposure to S1P.
Conclusion
Mature human thymocytes rely on S1P-R1 to migrate toward S1P. Taken in the context of murine work demonstrating that S1P is required for thymocyte egress to the periphery, our data highlight a new key chemokine for human thymocyte egress.
Keywords: Sphingosine-1-phosphate, thymic egress, S1P-R1, S1P-R2, T-cell reconstitution, migration, thymus, human immunology
Naive T cells develop in the thymus from CD34+ hematopoietic stem cells, which are produced in the bone marrow and enter the thymus from blood. Fewer than 5% of developing thymocytes survive positive and negative selection and exit as naive T cells at the thymic corticomedullary junction.1–3 Stages of thymocyte differentiation are characterized by changes in cell-surface antigens, such as an increase in expression levels of the CD3/T-cell receptor (TCR) complex and upregulation of CD27, CD45RA, and CD62 ligand (CD62L) on mature medullary thymocytes.4,5 In addition, transcriptional regulators, such as Kruppel-like factor 2 (KLF2), play an important role throughout thymocyte development by upregulating surface receptors necessary for migration throughout and egress from the thymus.6
Because little is known of factors that influence the egress of naive human T cells to the periphery, we examined sphingosine-1-phosphate (S1P) and its receptors, which have been shown to play essential roles in T- and B-lymphocyte migration in the mouse.7–9
S1P is a chemotactic sphingolipid molecule with varied roles throughout the body, many of which have been characterized exclusively in murine models.10–12 S1P is highly concentrated in the blood, where it is produced by erythrocytes, endothelial cells, and activated platelets,10,13,14 but levels are low in lymphoid tissues, where it is degraded by sphingosine lyase.15 Sphingosine kinase and sphingosine lyase maintain the S1P gradient, which promotes the influx of lymphocytes bearing one of its 5 G protein–coupled receptors (sphingosine-1-phosphate receptors [S1P-Rs] 1–5)10,16,17 to the lymph nodes. In the mouse, CD69, an activation marker expressed during thymic selection, has to be downregulated before thymocyte egress.18,19 CD69 is also downregulated on human mature thymocytes before thymic egress and is absent on recent thymic emigrants.20
S1P/S1P-R1 ligation in the mouse is required for mature naive T cells to egress from the thymus and for memory T cells to egress from secondary lymphoid tissues.7,19 Mice with S1p1−/− hematopoietic stem cells have virtually no naive T cells in the blood,7 and treatment of mice with FTY720, an S1P-R modulator,21 recapitulates this effect by triggering internalization of S1P-R1 and hence desensitizing T cells.7,22 In contrast, S1P-R2 inhibits migration of maturing B cells in the germinal centers when ligated to S1P9 and retains T follicular helper cells in the germinal centers.23 However, no studies exist demonstrating the role of S1P and its receptors in human thymocyte egress.
Here we show for the first time that the S1P/S1P-R1 axis plays a crucial role in human thymocyte egress and identify the phenotype of S1P-R1+ thymocytes as CD3hiCD69− cells within the CD27+CD45RA+ medullary thymocyte subset. Mature CD3hiCD69−, but not CD3hiCD69+, human thymocytes migrate toward S1P in vitro. Expression of S1P-R2 shows donor variability, but our data suggest that it might play an opposite inhibitory role to S1P-R1. Other S1P-R expression is either negligible throughout all stages of thymocyte development or is not significantly different across thymocyte populations, supporting our hypothesis that S1P-R1 is the principal receptor for S1P-mediated thymocyte egress.
METHODS
See the Methods section in this article’s Online Repository at www.jacionline.org for more details.
Compliance
The article submitted by the authors for publication meets all of the requirements for research with human fetal material, as defined by applicable Federal laws and the protection of human subjects, including:
Public Law 103–43, National Institutes of Health (NIH) Revitalization Act of 1993, also delineated in the NIH, Reminder of Legal Requirements Regarding the Acquisition and Use of Human Fetal Tissue for Research Purposes, and
Federal Regulations for the Protection of Human Subjects at Title 45 Code of Federal Regulations Part 46 (45 CFR 46).
Tissue collection and thymocyte preparation
Postnatal thymus specimens were obtained from children undergoing corrective cardiac surgery at Mattel Children’s Hospital at UCLA. Fetal thymus specimens were obtained from the UCLA CFAR Gene and Cellular Therapy Core. Thymocytes were prepared and cultured, as described previously.24
Flow cytometry and cell sorting
Surface immunophenotyping of thymocytes, either untreated or after exposure to 100 nmol/L S1P (Sigma, St Louis, Mo), was performed, as previously described,25,26 by using mAbs to S1P-R1 (or IgG2b control), CD45RA, CD4, CD8, CD62L, CD3, CD69, and CD27 (see Tables E1 and E2 in this article’s Online Repository at www.jacionline.org). For cell sorting, thymocytes were stained, as previously described.27
Quantitative real-time PCR
TaqMan reverse transcription quantitative PCR was performed to determine expression of the S1PR1 to S1PR5 and KLF2 genes relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control.
S1P chemotaxis/migration assay
Migration of thymocytes to S1P was assayed, as described by Matloubian et al.7 Briefly, cells in serum-free medium were loaded in the upper chamber after treatment with 100 nmol/L FTY720 for 1 hour, where indicated, and allowed to transmigrate for 3 hours across 5-μm Transwell filters (Costar; Corning, Corning, NY) to S1P (Sigma) at the indicated concentrations with or without S1P-R modulators. Input thymocytes and cells collected in the lower chamber were stained with mAbs to surface markers (see Table E1) and acquired for a fixed amount of time.
Statistical analysis
Analyses were conducted with GraphPad Prism 6 software (GraphPad Software, La Jolla, Calif). Tests used to analyze the experiments are summarized in Table E3 in this article’s Online Repository at www.jacionline.org. A P value of .05 or less was considered significant. Data are reported as means ± SEMs.
RESULTS
Only mature CD3hiCD27+CD45RA+CD62L+ thymocytes lacking CD69 expression migrate toward S1P
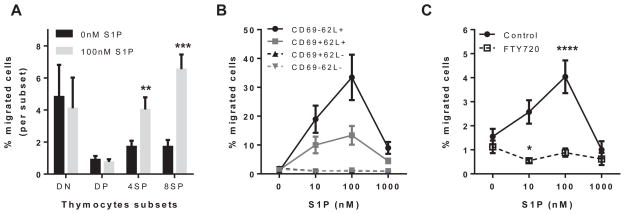
The function of the S1P/S1P-R system in human thymocytes was examined by using in vitro Transwell migration assays. Human postnatal thymocytes were added to the top chambers of Transwell plates, and S1P at the optimal concentration (100 nmol/L)7 was added to the bottom chambers. Migrated thymocytes were immunophenotyped to identify different stages of thymocyte development (see Table E1). As shown in Fig 1, A, immature CD4−CD8− double-negative (mostly CD3−) and CD4+CD8+ double-positive (mostly CD3lo) thymocytes did not migrate to S1P. In contrast, mature CD3hiCD27+ thymocyte subsets expressing either CD4 or CD8 did migrate to S1P (P < .001; Fig 1, A). These findings indicate that thymocytes responding to S1P are located in the thymic medulla (CD27+) and have completed positive selection and lineage commitment.
FIG 1.
Mature human thymocytes migrate to S1P. A, Percentage of immature CD3−CD4−CD8− (double-negative) and CD4+CD8+ (double-positive) and mature CD3hiCD27+ thymocyte subsets expressing CD4 or CD8 that migrate to 100 nmol/L S1P in a Transwell migration assay (total thymocytes in the upper chamber). B, Migration of mature CD3hiCD27+ thymocyte populations identified by using antibodies to CD69 and CD62L. C, Effect of FTY720, an S1P-R1, S1P-R3, S1P-R4, and S1P-R5 modulator, on migration of mature CD3hiCD27+ thymocytes. For statistical data, please see Table E3.
To further characterize the medullary thymocyte subsets that respond to S1P, we added antibodies to CD69 and CD62L (L-selectin) to our immunophenotyping panel. CD69 is an early activation molecule expressed during thymic selection but not present on thymocytes ready to egress or on recent thymic emigrants.18 CD62L is expressed on naive T cells and is important for homing to peripheral lymphoid tissues.28 We found that mainly mature CD3hiCD27+CD62L+ thymocytes that have lost CD69 expression migrate to S1P (Fig 1, B). No migration to S1P was observed in CD62L− thymocyte subsets. We tested a range from 0 to 1000 nmol/L S1P and found that cell migration peaked at 100 nmol/L (Fig 1, B), whereas at 1000 nmol/L, thymocytes became nonrespondent after a typical bell-shaped dose-response curve.29
S1P-R1 and potentially S1P-R2 control human thymocyte egress
Next, we used FTY720, a nonselective modulator of S1P-R1, to verify involvement of the S1P-Rs in S1P-promoted migration. FTY720 mimics S1P and binds to S1P-Rs, resulting in S1P-R1 internalization30 and precluding migration because of a lack of functional intracellular signaling. Mature CD3hiCD27+ thymocytes pretreated with 100 nmol/L FTY720 displayed impaired migration compared with untreated thymocytes (Fig 1, C). Untreated mature CD4 single-positive (SP) and CD8 SP thymocytes displayed approximately 4% and 7% migration, respectively, whereas less than 1% of FTY720-treated cells migrated toward S1P (data not shown). Interestingly, the migration of FTY720-treated CD4 SP thymocytes and, to a lesser extent, CD8 SP thymocytes was consistently reduced at 10 nmol/L S1P compared with the basal level of migration without S1P (P < .01 [t test] between 0 and 10 nmol/L S1P for the migration of CD4 SP thymocytes after treatment with FTY720, data not shown). Because FTY720 prevents S1P-R1 signaling, these results strongly suggest that stimulation of another S1P-R, such as S1P-R2 (see below), can inhibit migration to S1P.
S1P-R1 is the main S1P-R expressed in the thymus, and S1P-R1 mRNA is expressed to the greatest extent within the most mature CD3hiCD27+CD69−CD45RA+CD62L+ thymocyte subset
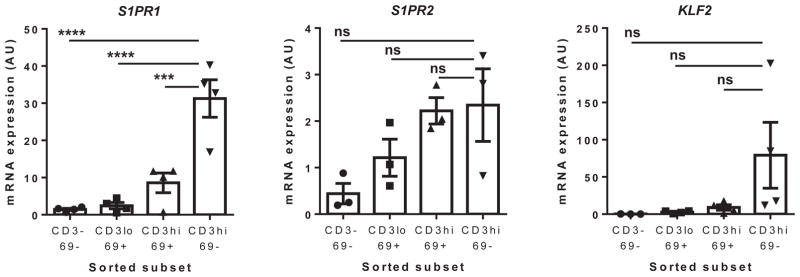
To determine at which stages of thymocyte maturation S1P-Rs are expressed, we sorted total postnatal thymocytes into 4 populations based on maturation phenotype: CD3−CD27−CD69−, CD3lo CD27−CD69+, CD3hiCD27+CD69+, and CD3hiCD27+CD69− (see Fig E1 in this article’s Online Repository at www.jacionline.org for the gating scheme). We and others have shown that the CD3hiCD69− subset is comprised of mature thymocytes presumably prepared to egress the thymus for the periphery,20,31 leading to our hypothesis that this subset would express S1P-R1 to the greatest extent. Considering the retentive role of S1P-R2 in murine B cells and follicular helper T cells within germinal centers, we hypothesized that S1P-R2 might have an analogous role in thymocytes.23,32 Quantitative real-time PCR for S1P-Rs 1 to 5 revealed that S1P-R1 is expressed to a significantly greater extent within the most mature CD3hiCD27+CD69− subset than the CD3hiCD27+CD69+ subset (>3-fold increase, P = .01), which represents the preceding stage of thymocyte development (Fig 2). Moreover, we found that S1PR1 was the main S1P-R mRNA expressed in human thymocytes because expression of S1PR2, S1PR4, and S1PR5 mRNA was quite low (Fig 2 and see Fig E2 in this article’s Online Repository at www.jacionline.org). In addition, S1PR3, S1PR4, and S1PR5 do not show variable expression in thymocyte subsets (see Fig E2) and do not appear to have a major role in egress based on migration assays using a specific antagonist to S1P-R1, W146 (data not shown).
FIG 2.
S1PR1 and KLF2 mRNA are expressed to the greatest extent within the most mature CD3hiCD69− thymocyte subset. Thymocytes were sorted into 4 populations based on maturation immunophenotype (ie, CD3−CD69−, CD3loCD69+, CD3hiCD69+, and CD3hiCD69−) before real-time PCR. S1PR1, S1PR2, and KLF2 mRNA are shown. For statistical data, please see Table E3.
In accordance with the above results, we hypothesized that S1P-R2 expression would be concentrated within the CD3hiCD69+ subset of thymocytes, fitting with its potential role in retaining maturing T cells in the thymus until complete maturation. Despite important donor variability (Fig 2), S1PR2 mRNA expression tended to be higher in the more mature subsets (CD3hiCD27+), with the lowest mRNA level consistently in the least mature CD3−CD27−CD69− thymocytes. The donor variability of S1PR2 mRNA was corroborated by inconsistent results in migration assays in which we used JTE-013, a specific S1P-R2 antagonist, to elucidate the role of this receptor (data not shown). Therefore we cannot draw definitive conclusions on the effect of S1P-R2 expression on migration into the thymus.
Kruppel-like factor 2 (KLF2) is a purported master transcriptional regulator of S1PR1 mRNA expression and regulator of other receptors involved in thymocyte development and egress of mature thymocytes to the periphery.33–35 Because KLF2 regulates S1PR1 expression in peripheral T cells,6,34–37 it follows that it might play a similar role in the human thymus. Thus we hypothesized that the highest S1P-R1–expressing subset should correspond to the subset expressing the greatest amount of KLF2 mRNA. Indeed, KLF2 and S1PR1 transcripts increase concurrently, with the lowest expression observed in CD3−CD69− (immature) and the highest expression in CD3hiCD69−CD27+ thymocytes (Fig 2).
S1P-R1 is expressed at the protein level on mature CD3hiCD69−CD27+CD45RA+CD62L+ thymocytes
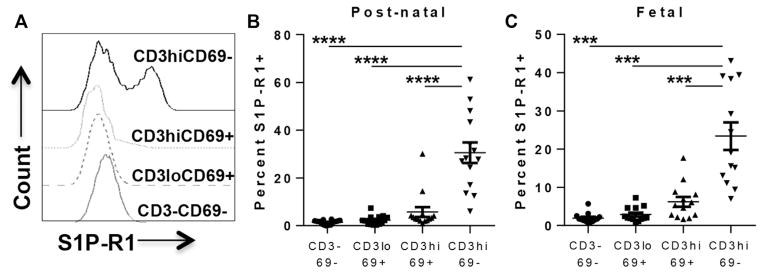
Human postnatal thymocytes (0 day to 3 years of age; median, 9 months; 14 donors) were stained with antibodies to S1P-R1 and CD3, CD27, CD45RA, CD62L, CD69, CD4, and CD8. To elucidate the immunophenotype of S1P-R1+ thymocytes, we first gated on the S1P-R1+ thymocytes and found that this population was mainly mature CD3hi, CD27hi, CD4 SP or CD8 SP, CD45RA+, CD62L+, and CD69− (see Fig E3, A, and for total thymocyte phenotype, see Fig E3, B, in this article’s Online Repository at www.jacionline.org). The S1P-R1+ thymocytes lacking CD3 are likely thymic B cells because we identified a CD3−CD19+CD45RA+S1P-R1+ population and, to a lesser extent, natural killer cells (CD3−CD19−CD45RA+S1P-R1+; see Fig E4 in this article’s Online Repository at www.jacionline.org).38 We then compared S1P-R1 expression in similar populations as those above for cell sorting and mRNA quantitation (see Fig E1 for gating). Confirming the quantitative real-time PCR results (Fig 2), we observed S1P-R1 expression predominantly on the CD3hiCD27+CD69− thymocyte population (Fig 3, A) and minimal S1P-R1 expression on all other subsets. S1P-R1 was expressed on approximately 30% of CD3hiCD69− thymocytes (n = 15; range, 5% to 60%) compared with only 1% to 5% of the 3 less mature populations (Fig 3, B). We further examined S1P-R1 expression within the mature CD3hiCD27+CD62L+ thymocytes on the CD45RA+CD69− subset and found S1P-R1 expression to be significantly higher within this subset than in CD45RA+CD69+ and CD45RA− thymocytes (P < .01, data not shown).
FIG 3.
S1P-R1 protein is expressed to the greatest extent on the most mature thymocyte subset. Human postnatal thymocytes were stained with surface antibodies (see Table E1). A, S1P-R1 profile of 1 representative postnatal (2-year-old) thymus gated as described above. B, Summary of S1P-R1 expression on cells from 14 postnatal thymi (median age, 9 months) showing comparison of S1P-R1 expression on CD27+CD3hiCD69− thymocytes to less mature populations. C, Expression of S1P-R1 on fetal thymocyte populations (median age, 16.5 weeks of gestation). For statistical data, please see Table E3.
To compare S1P-R1 expression on fetal with that on postnatal thymocytes, fetal thymocytes (14–21 weeks of gestation; median, 16.5 weeks; n = 12) were stained and analyzed the same way as described for postnatal cells. S1P-R1 expression on fetal thymocyte subsets (Fig 3, C) was similar to that on postnatal thymocytes (Fig 3, B) across the 4 populations of increasing maturation. Specifically, S1P-R1 expression on fetal thymocytes was 24% within CD3hiCD69− and 7% within CD3hiCD69+ cells (vs 30% and <5% on postnatal cells, respectively).
Thymocytes internalize S1P-R1 and downregulate S1PR1 and KLF2 mRNA in response to S1P
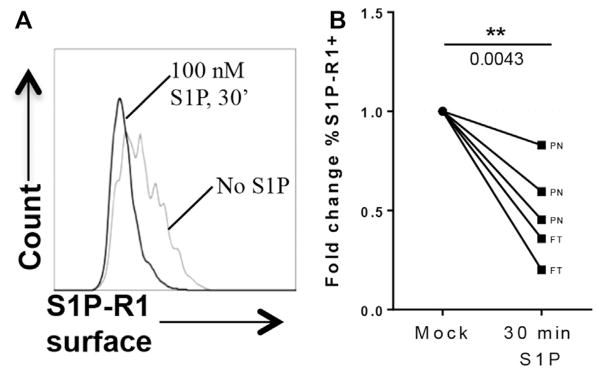
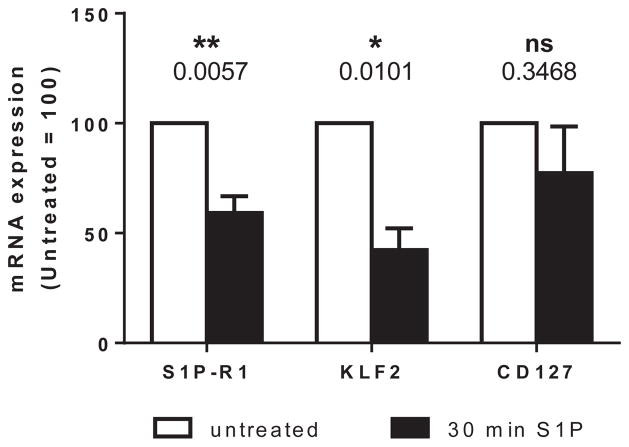
Because little is known about the regulation of S1P-R1 in the human thymus, we performed a series of in vitro S1P exposure assays and examined the effect on receptor expression. It has been reported that S1P/S1P-R ligation results in S1P-R1 internalization and initiation of intracellular signaling in naive CD4 and central memory CD4/CD8 T cells.39 In accordance with these data, we observed that mature human thymocytes, irrespective of origin (fetal or postnatal), displayed significantly reduced S1P-R1 surface expression by 30 minutes of S1P exposure, which is consistent with internalization and initiation of downstream signaling (Fig 4). In addition, we looked at the mRNA expression levels to determine whether receptor ligation affected its expression at the transcriptional level. Thymocytes prepared from human fetal thymic implants in immunodeficient mice, which are similar in phenotype to fetal and postnatal thymocytes,40 were exposed for 30 minutes to 100 nmol/L S1P and analyzed for S1PR1 mRNA expression. After exposure to S1P, S1PR1 mRNA expression was significantly downregulated relative to that in untreated donor-matched thymocytes (41% reduction, P = .0057). Interestingly, KLF2 mRNA was also significantly downregulated (58% reduction, P = .0101) after exposure to S1P (Fig 5). Concomitantly decreasing KLF2 and S1PR1 expression after S1P exposure is reminiscent of our observed trend of increasing KLF2 expression with increasing S1PR1 expression in developmental stages of thymocytes and supports a potential role for KLF2 as a regulatory factor of S1PR1 expression in human thymocytes, as reported in murine models.41 IL-7 receptor (IL7R) mRNA expression in the same donor-matched samples was not significantly altered (P = .3468) after 30 minutes of exposure to 100 nmol/L S1P, confirming the specificity of S1PR1 and KLF2 downregulation.
FIG 4.
Expression of S1P-R1 in mature CD3hiCD4+CD69− thymocytes after stimulation with S1P. Postnatal thymocytes were exposed to 100 nmol/L S1P in serum-free medium for 30 minutes or untreated and immediately stained for S1P-R1 and other surface markers. A, Change in surface expression of S1P-R1 between thymocytes exposed to S1P (black) versus medium (mock, light gray). B, Fold change in S1P-R1 expression after 30 minutes of S1P exposure of postnatal (PN) and fetal (FT) thymocytes. For statistical data, please see Table E3.
FIG 5.
S1PR1 and KLF2 mRNA expression decrease after exposure of fetal thymocytes to S1P. Thymocytes from human fetal thymus/liver implants in immunodeficient mice were exposed to 100 nmol/L S1P or medium alone for 30 minutes and subsequently analyzed for S1PR1 and KLF2 mRNA expression. Fold change of S1PR1, KLF2, and IL7R mRNA after S1P exposure is depicted. For statistical data, please see Table E3.
DISCUSSION
For the first time, we describe expression and function of the S1P-Rs in the human thymus. Before our research, there were very limited data on the mechanisms regulating egress of mature human thymocytes from the thymus to the periphery. Studies in the mouse examined the requirement for S1P-R1 in thymocyte egress and showed that S1P-R1 is essential for the egress of mature murine thymocytes.7,19 However, no reports to date have demonstrated whether the S1P/S1P-R pathway plays a role in the response of human mature thymocytes to S1P or egress from the human thymus to the peripheral blood and lymphoid tissues; importantly, the exact population of thymocytes that expresses S1P-R1 was not characterized in either mice or human subjects.
Our studies present an essential role for S1P-R1 in the human thymus. We show that S1P-R1 is expressed by CD3hiCD27+CD45RA+CD69−CD62L+ thymocytes and that the cells in this subset respond to S1P in vitro. This phenotype corresponds to the most mature thymocytes ready for egress to the periphery.20,42 We previously reported that CD69 is expressed on medullary thymocytes during the positive selection stage43 but lost before egress of mature naive T cells.20 Thus loss of CD69 is required for thymocyte response to S1P. Furthermore, IFN-α–induced CD69 expression has been shown to negatively regulate S1P-R1,19 and we have reported that plasmacytoid dendritic cells localized in the thymic medulla constitutively produce IFN-α.24,27 These arguments support the notion that CD69 might negatively regulate S1P-R1 expression throughout human thymocyte development in an IFN-α–dependent manner until immediately before egress.
KLF2 mRNA expression increased throughout the 4 human thymocyte developmental stages concomitant to the S1PR1 mRNA increase. In mice KLF2 is transiently expressed throughout thymocyte development and upregulated along with S1P-R1 in the mature CD3hiCD4+ and CD8+ thymocytes before thymocyte egress.16 KLF2 is a master transcriptional regulator dictating S1P-R1 expression34,36,37 and also regulates CD62L,6 which is expressed immediately before thymocyte egress. In the mouse KLF2 expression is regulated by forkhead box protein O1 (FOXO1), the activity of which is controlled through Akt phosphorylation. KLF2 modulation is likely related to TCR signaling during positive and negative selection. Because TCR signaling decreases in the ultimate stages of thymopoiesis, FOXO1, in a nonphosphorylated state, can bind to and activate the KLF2 gene promoter.44 KLF2 then activates the transcription of egress-associated S1PR1 and CD62L.
Because the majority of investigation in this area has been performed in mice, we decided to investigate the dynamics of S1P-R1/S1P binding and the molecular regulation of the S1PR1 gene in the human thymus. We found that when mature human thymocytes (CD3hiCD27+CD45RA+CD69−) expressing S1P-R1 were exposed to S1P, S1P-R1 was internalized. In addition, S1PR1 and KLF2 transcripts decreased upon exposure of human thymocytes to S1P. Altogether, our results support a role for KLF2 in activating S1P-R1 expression in the human thymus in a TCR signaling–dependent manner. These findings also imply that S1P-R1 transcription is regulated by a negative feedback mechanism that is initiated on S1P/S1P-R1 ligation and subsequent signaling and likely involves KLF2. Therefore KLF2 might be a potential key regulator of the timing of human thymocyte egress, with a potential role for FOXO1 in the regulation of the transcription of KLF2 and thereby S1PR1.
Levels of S1PR2 mRNA peak in the CD3hiCD69+ semimature subset and remain stable in the CD3hiCD69− mature subset (Fig 2). After FTY720-induced internalization of S1P-R1, we observed a reduction in the basal level of migration of mature thymocytes. Interestingly, this behavior was mostly present among CD4 SP cells rather than CD8 SP cells. Because S1P-R2 is the only other S1P-R showing significant expression on thymocytes and considering the recently reported role that S1P-R2 plays in confining murine germinal center B cells to the follicle45 and retaining follicular helper T cells in the germinal center,23 these results suggest that the function of S1P-R2 in the human thymus is to retain maturing cells throughout the final stages of selection. It was recently reported that FTY720 actually signals through S1P-R2 in myofibroblasts.21 Therefore it is possible that the deepest decrease in migration (10 nmol/L S1P) for CD4 SP resulted from S1P-R2 signaling induced by FTY720 and S1P. Altogether, these results suggest that S1P-R2 might play a role in thymocyte retention. A hypothetical mechanism is as follows: S1P-R2 signaling begins on CD3hiCD69+ thymocytes before S1P-R1 is expressed; then, because S1P-R1 expression increases on mature CD3hiCD69− cells, signaling through S1P-R1 takes precedence over S1P-R2 signaling, and naive T cells can follow the S1P gradient and egress.
In conclusion, our results suggest that S1P-R1 plays a major role in thymocyte egress, and S1P-R2 might be involved as well. Before this work, it had not been reported whether human thymocytes expressed S1P-Rs or responded to S1P. Our data show that in the human thymus only phenotypically mature cells expressing S1P-R1 are able to migrate to S1P and highlight a new key requirement for human thymocyte egress.
In addition to its crucial role in thymocyte egress, the S1P/S1P-R1 axis might have significant implications for immunodeficiencies, as well as lymphoproliferative and other diseases, in human subjects16,30,46 when considered in the context of the thymus. In the case of immunodeficiencies, investigation of the potential to modulate S1P and its receptors in the thymus is warranted. Increasing thymic output of subjects with low T-cell counts or function without increasing the risk of autoimmunity is an interesting potential therapeutic option. HIV-infected individuals failing to reconstitute adequate functional T cells or patients after bone marrow transplantation could benefit from this approach. On the other hand, dampening the response to S1P, perhaps through novel intrathymic use of receptor modulators, might prove useful in patients with lymphoproliferative diseases, such as certain lymphomas. Administration of FTY720 (fingolimod) has already been successful in the treatment of multiple sclerosis,47 abrogation of asthma,48 and enhancement of bone marrow allograft incorporation.49,50 The potential for local modulation of the mechanisms of T-cell egress is, as yet, unexplored in the thymus and represents an intriguing new possibility.
Supplementary Material
Key Messages.
S1P-R1 is the main S1P receptor expressed on mature human thymocytes (CD3hiCD27+CD69−) ready to egress from the thymus to the periphery.
For the first time we characterize the S1P-R1+ human thymocyte population as CD3hiCD27+CD45RA+CD69− CD62L+ cells.
S1P-R1 expression mediates the response of mature human thymocytes to S1P.
In agreement with published murine data, we found that S1P-R1/S1P ligation is likely required for human thymocyte egress.
The data highlight a key new requirement for human thymocyte egress and have potential implications for novel immunomodulatory therapies as we show that S1P-Rs on human thymocytes can be modulated with chemical mediators.
Acknowledgments
Supported by grants from the National Institutes of Health (NIH; R21 AI102771 and R01 AI080564 to C.H.U.) and by the California HIV/AIDS Research Program (CHRP), Dissertation Fellowship (D13-LA-394), and NIH T32 (5T32HL086345-08; to R.S.R.). The services of the UCLA CFAR Cores for Flow Cytometry and Virology were supported by NIH-AI28697, and those of the UCLA Tissue Procurement Core were supported by NIH-CA-16042.
We acknowledge the services of the UCLA CFAR Cores for Flow Cytometry and Virology supported by NIH-AI28697 and the UCLA Tissue Procurement Core supported by NIH-CA-16042.
Abbreviations used
- CD62L
CD62 ligand
- FOXO1
Forkhead box protein O1
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- KLF2
Kruppel-like factor 2
- NIH
National Institutes of Health
- SP
Single-positive
- S1P
Sphingosine-1-phosphate
- S1P-R1
Sphingosine-1-phosphate receptor
- TCR
T-cell receptor
Footnotes
Disclosure of potential conflict of interest: R. S. Resop has received research support from the National Institutes of Health (NIH; T32 Developmental Hematology, T32HL086345), California HIV/AIDS Research Program (CHRP) Graduate Fellowship (D13-LA-394), the NIH/NIAID (R21 AI102771 and R01 AI080564), and has received travel support from the AAI. M. Douaisi has received research support from NIH/NIAID (RO1 AI080564) and is employed by Rensselaer Polytechnic Institute (Troy, NY). J Craft has received research support from the NIH/NIAID (RO1 AI08564). L. C. M. Jachimowski and B. Blom have received research support from the NIH/NIAID (R21 AI102771 and R01 AI080564). C. H. Uittenbogaart has received research support from the NIH/NIAID (R21 AI102771 and R01 AI080564).
References
- 1.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–22. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 2.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–35. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 3.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–91. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 5.Ho Tsong Fang R, Colantonio AD, Uittenbogaart CH. The role of the thymus in HIV infection: a 10 year perspective. AIDS. 2008;22:171–84. doi: 10.1097/QAD.0b013e3282f2589b. [DOI] [PubMed] [Google Scholar]
- 6.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 7.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 8.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–33. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–20. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 10.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–63. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel S, Cuvillier O, Edsall L, Kohama T, Menzeleev R, Olivera A, et al. Roles of sphingosine-1-phosphate in cell growth, differentiation, and death. Biochemistry (Mosc) 1998;63:69–73. [PubMed] [Google Scholar]
- 12.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–4. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi Y, Yatomi Y. Sphingosine 1-phosphate is a blood constituent released from activated platelets, possibly playing a variety of physiological and pathophysiological roles. Acta Biochim Pol. 1998;45:299–309. [PubMed] [Google Scholar]
- 14.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997;121:969–73. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 15.Veltkamp R, Siebing DA, Heiland S, Schoenffeldt-Varas P, Veltkamp C, Schwaninger M, et al. Hyperbaric oxygen induces rapid protection against focal cerebral ischemia. Brain Res. 2005;1037:134–8. doi: 10.1016/j.brainres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–15. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531:54–7. doi: 10.1016/s0014-5793(02)03480-4. [DOI] [PubMed] [Google Scholar]
- 18.Feng C, Woodside KJ, Vance BA, El-Khoury D, Canelles M, Lee J, et al. A potential role for CD69 in thymocyte emigration. Int Immunol. 2002;14:535–44. doi: 10.1093/intimm/dxf020. [DOI] [PubMed] [Google Scholar]
- 19.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–4. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 20.Vanhecke D, Leclercq G, Plum J, Vandekerckhove B. Characterization of distinct stages during the differentiation of human CD69+CD3+ thymocytes and identification of thymic emigrants. J Immunol. 1995;155:1862–72. [PubMed] [Google Scholar]
- 21.Sobel K, Monnier L, Menyhart K, Bolinger M, Studer R, Nayler O, et al. FTY720 Phosphate activates sphingosine-1-phosphate receptor 2 and selectively couples to Galpha12/13/Rho/ROCK to induce myofibroblast contraction. Mol Pharmacol. 2015;87:916–27. doi: 10.1124/mol.114.097261. [DOI] [PubMed] [Google Scholar]
- 22.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 23.Moriyama S, Takahashi N, Green JA, Hori S, Kubo M, Cyster JG, et al. Sphingosine-1-phosphate receptor 2 is critical for follicular helper T cell retention in germinal centers. J Exp Med. 2014;211:1297–305. doi: 10.1084/jem.20131666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurney KB, Colantonio AD, Blom B, Spits H, Uittenbogaart CH. Endogenous IFN-alpha production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV-1 infection. J Immunol. 2004;173:7269–76. doi: 10.4049/jimmunol.173.12.7269. [DOI] [PubMed] [Google Scholar]
- 25.Uittenbogaart CH, Anisman DJ, Zack JA, Economides A, Schmid I, Hays EF. Effects of cytokines on HIV-1 production by thymocytes. Thymus. 1994;23:155–75. [PubMed] [Google Scholar]
- 26.Schmid I, Uittenbogaart CH, Giorgi JV. Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry. 1994;15:12–20. doi: 10.1002/cyto.990150104. [DOI] [PubMed] [Google Scholar]
- 27.Colantonio AD, Epeldegui M, Jesiak M, Jachimowski L, Blom B, Uittenbogaart CH. IFN-alpha is constitutively expressed in the human thymus, but not in peripheral lymphoid organs. PLoS One. 2011;6:e24252. doi: 10.1371/journal.pone.0024252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munro JM, Briscoe DM, Tedder TF. Differential regulation of leucocyte L-selectin (CD62L) expression in normal lymphoid and inflamed extralymphoid tissues. J Clin Pathol. 1996;49:721–7. doi: 10.1136/jcp.49.9.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohno T, Igarashi Y. Attenuation of cell motility observed with high doses of sphingosine 1-phosphate or phosphorylated FTY720 involves RGS2 through its interactions with the receptor S1P. Genes Cells. 2008;13:747–57. doi: 10.1111/j.1365-2443.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- 30.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Res P, Blom B, Hori T, Weijer K, Spits H. Downregulation of CD1 marks acquisition of functional maturation of human thymocytes and defines a control point in late stages of human T cell development. J Exp Med. 1997;185:141–51. doi: 10.1084/jem.185.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green JA, Cyster JG. S1PR2 links germinal center confinement and growth regulation. Immunol Rev. 2012;247:36–51. doi: 10.1111/j.1600-065X.2012.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart GT, Hogquist KA, Jameson SC. Kruppel-like factors in lymphocyte biology. J Immunol. 2012;188:521–6. doi: 10.4049/jimmunol.1101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178:7632–9. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- 35.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 36.Hogquist KA, Weinreich MA, Jameson SC. T-cell migration: Kruppeled T cells move again. Immunol Cell Biol. 2008;86:297–8. doi: 10.1038/icb.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinreich MA, Hogquist KA. Thymic emigration: when and how T cells leave home. J Immunol. 2008;181:2265–70. doi: 10.4049/jimmunol.181.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–81. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mudd JC, Murphy P, Manion M, Debernardo R, Hardacre J, Ammori J, et al. Impaired T-cell responses to sphingosine-1-phosphate in HIV-1 infected lymph nodes. Blood. 2013;121:2914–22. doi: 10.1182/blood-2012-07-445783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uittenbogaart CH, Boscardin WJ, Anisman-Posner DJ, Koka PS, Bristol G, Zack JA. Effect of cytokines on HIV-induced depletion of thymocytes in vivo. AIDS. 2000;14:1317–25. doi: 10.1097/00002030-200007070-00003. [DOI] [PubMed] [Google Scholar]
- 41.Shannon LA, McBurney TM, Wells MA, Roth ME, Calloway PA, Bill CA, et al. CCR7/CCL19 controls expression of EDG-1 in T cells. J Biol Chem. 2012;287:11656–64. doi: 10.1074/jbc.M111.310045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–8. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- 43.Gurney KB, Uittenbogaart CH. Human immunodeficiency virus persistence and production in T-cell development. Clin Vaccine Immunol. 2006;13:1237–45. doi: 10.1128/CVI.00184-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Love PE, Bhandoola A. Signal integration and crosstalk during thymocyte migration and emigration. Nat Rev Immunol. 2011;11:469–77. doi: 10.1038/nri2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green JA, Suzuki K, Cho B, Willison LD, Palmer D, Allen CD, et al. The sphingosine 1-phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol. 2011;12:672–80. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oskeritzian CA. Mast cell plasticity and sphingosine-1-phosphate in immunity, inflammation and cancer. Mol Immunol. 2015;63:104–12. doi: 10.1016/j.molimm.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkmann V, Pinschewer DD, Feng L, Chen S. FTY720: altered lymphocyte traffic results in allograft protection. Transplantation. 2001;72:764–9. doi: 10.1097/00007890-200109150-00002. [DOI] [PubMed] [Google Scholar]
- 48.Idzko M, Hammad H, van Nimwegen M, Kool M, Muller T, Soullie T, et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116:2935–44. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrie Aronin CE, Shin SJ, Naden KB, Rios PD, Jr, Sefcik LS, Zawodny SR, et al. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials. 2010;31:6417–24. doi: 10.1016/j.biomaterials.2010.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang C, Das A, Barker D, Tholpady S, Wang T, Cui Q, et al. Local delivery of FTY720 accelerates cranial allograft incorporation and bone formation. Cell Tissue Res. 2012;347:553–66. doi: 10.1007/s00441-011-1217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.