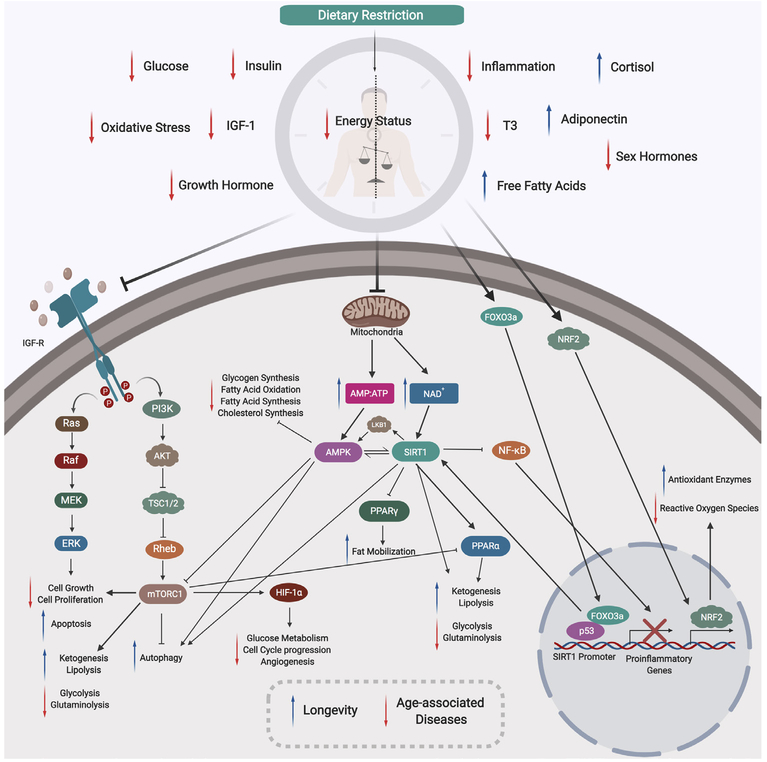
Figure 2.
Mechanism of Dietary Restriction. During DR there is a reduced energy status within the organism, resulting in corresponding decreases in blood glucose, insulin, IGF-1, growth hormones, sex hormones, and T3 thyroid hormones. Also, DR induces a reduction in both oxidative stress and inflammation. Following DR, there are increases in free fatty acids, adiponectin, and cortisol. A lower cellular energy status will lead to reduced mitochondrial activity and aerobic respiration, increasing the AMP:ATP ratio and NAD+ levels. Two major cellular nutrients and energy sensors, AMPK and SIRT1, will be then be activated, respectively. Activated AMPK will inhibit glycogen synthesis, ACC1, ACC2, and HMG-CoA to drive a reduction in fatty acid synthesis, oxidation, and cholesterol synthesis. Activated SIRT1 can enhance ketogenesis and lipolysis, and downregulate glycolysis and glutaminolysis. These effects may be also due to an activation of PPARα, reflecting direct and indirect functions of SIRT1. Activated SIRT1 can also repress the activity of PPARγ to modulate lipid metabolism and enhance fat mobilization in white adipose tissue during DR. Activated SIRT1 can inhibit NF-κB activity, inhibiting the expression of proinflammatory genes. SIRT1 activation is dependent on the increased interaction between FOXO3a and p53 at SIRT1 promoter sites. The physical interaction between FOXO3a and p53, and the subsequent activation of SIRT1, play important roles in repressing cell growth and proliferation. Notably, SIRT1 activation can activate LKB1, which will further activate AMPK, thus creating a positive feedback loop. Reduced levels of insulin, glucose and growth hormone downregulates insulin and IGF-R signaling pathways, inactivating the RAS/MAPK axis, repressing cell growth and proliferation and promoting apoptosis. Repression of IGF-R signaling will include the PI3K pathway, and inhibit downstream mTORC1, promoting autophagy. Inhibition of mTORC1 can also occur via activated AMPK in response to DR. Decreased mTORC1 function can promote ketogenesis and lipolysis, and repress glycolysis and glutaminolysis. Moreover, the corresponding decreased function of mTORC1 also inhibit HIF-1α, which downregulates key biological processes such as glucose metabolism, angiogenesis, and cell cycle progression. The NRF2 pathway is also triggered by DR, which helps to increase production of protective antioxidant enzymes and mitochondrial biogenesis to reduce ROS activity. With lower levels of ROS, there will be reduced DNA damage and a maintained genome stability. Lower energy status will prime cells to low-intensity stress, and this hormetic mechanism will stimulate better management of stress, upregulate DNA repair genes, as well as shifting towards a preferred setting of maintenance and repair (not shown). Overall, DR can act via these mechanisms to counter age-associated diseases and induce longevity.
DR, dietary restriction; IGF-1, insulin growth factor 1; AMP, adenosine monophosphate; ATP, adenosine triphosphate; NAD+, nicotinamide adenine dinucleotide; AMPK, adenosine monophosphate kinase; SIRT1, sirtuin 1 deacetylase; ACC1 and ACC2, acetyl-coenzyme A carboxylase 1 and 2; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A; PPARα and PPARγ, peroxisome proliferator-activated receptor gamma alpha and gamma; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; FOXO3a, forkhead box O3; LKB1, liver kinase B1; IGF-R, insulin growth factor receptor; RAS/MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; mTORC1, mammalian target of rapamycin complex 1; HIF-1α, hypoxia-inducible factor 1 alpha; NRF2, nuclear factor erythroid 2-related factor 2 ;ROS, reactive oxygen species.