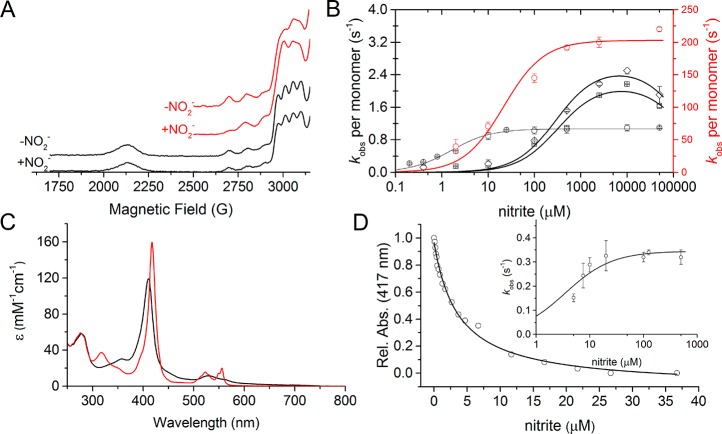
Figure 5.
Nitrite binding and redox communication between the tethered haem domain and the catalytic T2Cu in RpNiR. (A) EPR spectra of the different RpNiR constructs with and without 5 mM nitrite present. In (A), the WT RpNiR spectra is shown in black and the RpNiR-core is shown in red. (B) Steady state Michaelis–Menten plots of the different CuNiR constructs (WT AxNiR is shown as red circles; WT RpNiR is shown as black circles; RpNiR-core is shown as black squares; and Y323F RpNiR-core is shown as black diamonds). The kinetic parameters obtained are shown in Table 4. (C) UV–vis spectra of oxidized (black) and 1-electron reduced (red) WT RpNiR, showing how 1-electron predominately sits on the haem cofactor. (D) Effect of titrating nitrite into 1-electron reduced WT RpNiR measured by the absorbance of the haem Soret peak. The insert in (D) shows the observed rate constants for the reaction between 1-electron reduced RpNiR and nitrite. The Ks value for nitrite binding measured by UV–vis spectral titration is 3.7 × 10–3 ± 0.3 × 10–3 mM, and the Ks and klim values for the nitrite dependent stopped-flow are 3.5 × 10–3 ± 1.2 × 10–3 mM and 0.34 ± 0.02 s–1, respectively.