Figure 8.
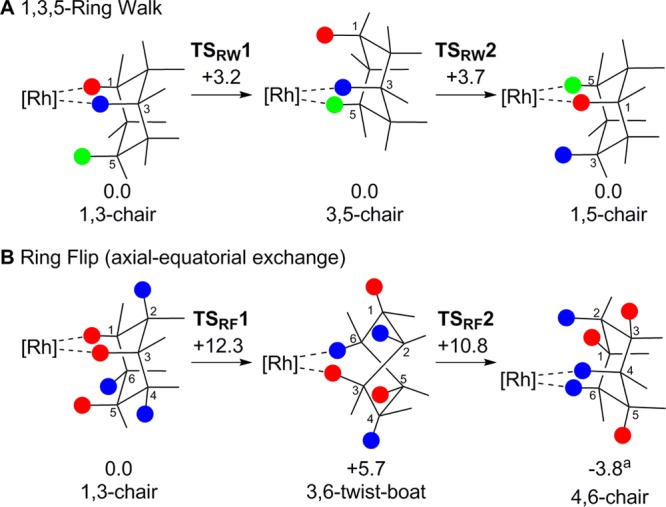
Computed pathways for cyclohexane rearrangements in [1-C6H12][BArF4] via (A) 1,3,5-ring walk and (B) ring flip mechanisms, with free energies indicated in kcal/mol. aThe SCF electronic energy of this 4,6-chair structure places it 2.1 kcal/mol above the 1,3-chair; however, a large stabilization due to thermodynamic corrections gives this anomalously low free energy.84
