Figure 3. Cystine can transfer disulfide bonds to proteins in vitro and in vivo.
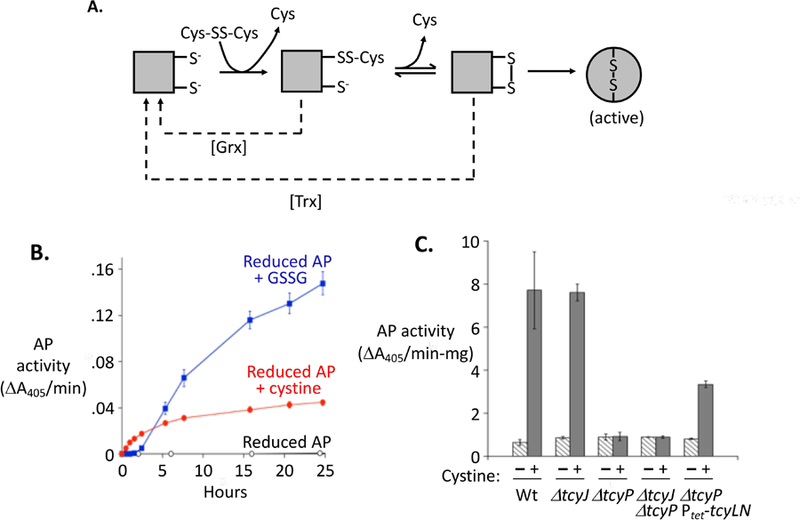
(A) Steps by which low-molecular-weight disulfide compounds can transfer disulfide bonds to alkaline phosphatase (AP), thereby activating it. AP ultimately folds around the disulfide bonds, making activation irreversible (Akiyama & Ito, 1993). For clarity, only one of the two disulfide bonds in AP is represented. Dashed lines: The glutaredoxin (Grx) and thioredoxin (Trx) systems suppress AP activation, likely by reducing mixed and the initial intra-protein disulfide bonds, respectively. (B) Cystine and oxidized glutathione (glutathione disulfide, GSSG) at 0.5 mM activate purified AP in vitro. (C) Cystine import activates cytoplasmic AP in vivo. Cystine was added (or not) to strains containing the pcAP plasmid, and intracellular AP activity was measured 40 min later. Strains bearing the pcAP plasmid are WP551 (WT); KCI1630 (ΔtcyJ); KCI1632 (ΔtcyP); KCI1820 (ΔtcyJ ΔtcyP); and KCI1638 (ΔtcyP Ptet-RAc-tcyLN), which constitutively overexpresses the integral membrane proteins of the TcyJLN cystine importer. Error bars in this and all other figures represent SEM of at least three biological replicates.
