Abstract
Background and objective
Patients with chronic respiratory failure are increasingly managed with domiciliary non-invasive ventilation (NIV). There may be limited ability to provide NIV titration for these complex patients, and ventilatory requirements and upper airway support needs may change over time. Therefore, an automatically adjusting expiratory positive airway pressure (AutoEPAP) algorithm may offer advantages over manually-adjusted EPAP for treating these patients. This study compared oxygen desaturation index (ODI 4%) values during use of an AutoEPAP algorithm versus manual EPAP titration with the intelligent volume-assured pressure support (iVAPS) algorithm.
Methods
This prospective, single-blind, randomized, crossover study was conducted at six US sites. Patients with chronic respiratory failure (neuromuscular disease, chronic obstructive pulmonary disease, obesity hypoventilation, and other etiologies) and an apnoea-hypopnoea index of >5/h who were already established NIV users underwent a single night of NIV with iVAPS manual EPAP and iVAPS AutoEPAP in the sleep lab, in random order.
Results
A total of 38 patients constituted the study population. Mean ODI4% was statistically non-inferior with AutoEPAP versus manual EPAP (p<0.0001). There was no difference in the effect on ODI4% across respiratory failure subgroups. Ventilation parameters and gas exchange were similar with either NIV mode, indicating equally effective treatment of respiratory failure. Sleep parameters were improved during AutoEPAP versus manual EPAP.
Conclusion
A single night of NIV using the iVAPS with AutoEPAP algorithm was non-inferior to a single night of iVAPS with manual EPAP titration in patients with respiratory failure.
Clinical Trial Registration
Keywords: non-invasive ventilation, neuromuscular disease, chronic obstructive pulmonary disease, obesity hypoventilation syndrome, respiratory failure
Introduction
Over recent years, increasing numbers of patients with neuromuscular disease (NMD), chronic obstructive pulmonary disease (COPD), obesity hypoventilation syndrome (OHS), and other forms of chronic respiratory failure are using non-invasive ventilation (NIV) for respiratory support 1. Optimization of settings for NIV, including determination of fixed expiratory positive airway pressure (EPAP), requires time-consuming manual titration by experts. Guidelines recommend the use of attended polysomnography (PSG) as part of this process; however, these resources are not always available. Titration of ventilation based on bedside evaluation is commonly practiced but has been noted to decrease the quality of sleep, and therefore is not ideal 2. In addition, prescriptions based on the current condition only reflect requirements on that single visit. A patient’s condition often changes over time due to disease progression, changes in body weight, alterations in prescribed medication, tissue edema, and variable posture 3–5.
Advances in technology demonstrates that NIV devices could meet changes in ventilation demand by automatically adjusting inspiratory pressure via the volume-assured pressure support (VAPS) algorithm. VAPS has already been examined for the treatment of chronic respiratory failure, OHS and NMD 6–11. There is a growing evidence that VAPS is as effective as manually titrated pressure support ventilation for treating respiratory insufficiency or failure 6, 10, 12–15.
Some conditions that could be treated with VAPS may also have a component of upper airway obstruction; patients might therefore benefit from automatically-determined EPAP. Such an AutoEPAP algorithm has recently been incorporated into the iVAPS mode to help maintain airway patency. A recent randomized clinical trial of the iVAPS AutoEPAP algorithm showed non-inferiority to fixed EPAP in patients with chronic hypoventilation 16, predominantly in patients with OHS and COPD who are not ventilator-dependent.
The goal of this study was to compare the ability of the AutoEPAP algorithm to manage upper airway obstruction, comparing with manual EPAP in iVAPS mode in chronic respiratory failure patients receiving NIV. The hypothesis was that the automatic settings determined by the AutoEPAP algorithm would be non-inferior to manual EPAP in preventing desaturation. We also aimed to examine the effect on ventilation parameters, gas exchange, and sleep quality.
Methods
Study design
This prospective, multicentre, single-blind, randomized, crossover, non-inferiority trial was conducted at six sites in the United States. The study protocol was approved by Western Institutional Review Board (IRB) and the IRBs at each participating centre, and patients provided written informed consent before enrolment in the trial. The trial was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. Study oversight and monitoring were provided by the ResMed Medical Affairs Department (San Diego, CA, USA). The study was registered at clinicaltrials.gov ().
Patients
Eligible patients were adults with documented chronic respiratory failure. Sleep hypoventilation was defined as those with historical transcutaneous carbon dioxide [TcCO2] increase of ≥10 mmHg) and/or daytime hypercapnia (PaCO2 >45 mmHg). In addition to hypoventilation, all subjects had previously documented sleep apnoea or an apnoea-hypopnoea index (AHI) ≥5/h from a diagnostic study or recorded from the patient’s NIV device. The rationale for this criteria was to include subjects with a range of upper airway collapsibility, which might be reflected in a prior sleep apnea diagnosis or otherwise with episodes of discreet respiratory events leading to an elevated AHI. Subjects had been using NIV in spontaneous-timed (ST) or volume-assured pressure support (VAPS) mode for ≥3 months and had EPAP settings reviewed within the previous 12 months. Exclusion criteria included noncompliance with NIV (average usage of <4 h/night), use of oxygen therapy ≥5 L/min, acute disease exacerbation requiring hospitalization with the last 3 months, acute illness or medical instability, untreated non-OSA sleep disorders, surgery of the upper airway, nose, sinus or middle ear within the previous 90 days, and inability to provide informed consent and/or comply with the study protocol.
Procedures
All participants completed two, overnight, in-lab polysomnography (PSG) studies; one each using manual EPAP and AutoEPAP in iVAPS mode on NIV (Astral™ 150, ResMed Corp), assigned in random order, usually on two consecutive nights. The subject was blinded to the intervention by shielding the display of the Astral ventilator. In order to perform titration, the titrating technician was aware of the intervention. All sites were instructed to set the NIV device in VAPS mode (iVAPS) as close to the patient’s current NIV settings as possible. On the night that the patient was put on iVAPS with AutoEPAP, sites were instructed to keep the range “open” so the algorithm could be tested across the full range (EPAP 5–15 cmH2O and pressure support [PS] 4–20 cmH2O). All sites and investigators were instructed to place each patient’s clinical need and safety first. Therefore, clinically required NIV setting adjustments were permitted. On the manual EPAP night, technicians titrated the EPAP according to American Academy of Sleep Medicine accredited laboratory standards, utilizing respiratory effort belts, snoring, and desaturations in the absence of flow signals 17. On each PSG night, TcCO2 was measured using a bedside monitor (SenTec Digital Monitor, SenTec). All PSG studies were scored by a registered polysomnographic sleep technologist without knowledge of the intervention according to American Academy of Sleep Medicine criteria by a central core laboratory at the University of California, San Diego, USA. In order to assure blinding of the scoring laboratory, no data from the Astral ventilator, mask pressures, or respiratory flow was recorded in the PSG software.
Outcomes
The primary outcome was the 4% oxygen desaturation index (ODI4%). Secondary objectives assessed whether the AutoEPAP algorithm was effective for treating respiratory failure, and included the device-reported AHI, TcCO2, and sleep parameters during use of NIV with AutoEPAP versus manual EPAP.
Sample size
The study was powered to demonstrate ODI4% non-inferiority between NIV with AutoEPAP versus manual EPAP based on a non-inferiority margin of 2. Sample size calculations assumed an expected mean difference in ODI4% of 0 with a standard deviation of 1.55, power of 80% and two-sided alpha of 0.05. Power calculations based on a paired two-sided t-test showed that 23 patients would be sufficient. To allow for dropouts and to maximize study power, it was decided to enroll up to 40 subjects.
Statistical analysis
All statistical analyses were generated using SAS version 9.3 or later. All programming code was independently peer reviewed for accuracy. Heterogeneity of response across sites was tested using analysis of variance (ANOVA) based on a two-sided p-value of 0.15.
The intention-to-treat (ITT) population included all randomized patients who began the first study PSG night. The evaluable population included all patients from the ITT population who fulfilled the inclusion/exclusion criteria and completed study assessments as defined in the protocol. All primary and secondary endpoint analyses were performed in the evaluable population, while safety analyses included the ITT population.
Descriptive statistics were calculated for continuous variables, and frequencies and percentages were calculated for categorical data. Tests for normality were generated for continuous variables, as appropriate, and additionally, data were inspected for symmetry and severe outliers. For paired comparisons of continuous variables, the distribution of the data was considered and in cases where the results were skewed, a Wilcoxon signed-rank or sign test was used, as appropriate. Otherwise, a paired t-test was utilized based on an alpha of 0.05.
The null (H0) and alternative (H1) hypotheses for the primary endpoint were based on a non-inferiority test using a non-inferiority margin (d) of 2 events/hour, where μA-B is the mean paired difference in ODI4% between AutoEPAP and manual EPAP:
H0: μA-B > d (AutoEPAP algorithm is inferior)
H1: μA-B ≤ d (AutoEPAP algorithm is non-inferior)
The primary hypothesis was tested using a one-sided paired t-test of the difference, and the 95% upper one-sided confidence bound for the mean paired difference in ODI4% between manual and Auto EPAP was calculated. A crossover analysis was performed to investigate the influence of a possible period effect on the primary endpoint.
Subgroup analyses were performed on the primary endpoint, based on disease type (COPD, neuromuscular disease, and OHS and other combined) and ventilator dependency (dependent vs non-dependent). An ANOVA was generated to compare differences in the primary endpoint between AutoEPAP versus manual EPAP across subgroups.
Results
Study population
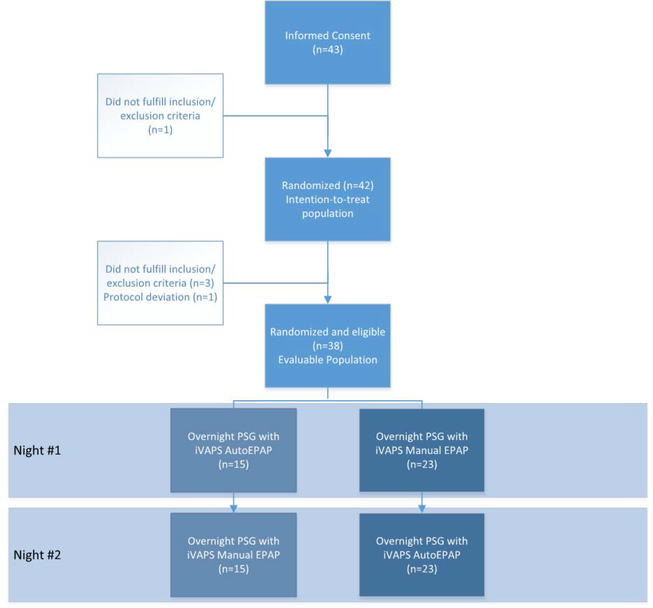
Between April 2016 and July 2017, a total of 43 patients were enrolled and provided informed consent; 42 of these were randomized and met the inclusion/exclusion criteria. The trial was stopped after recruitment goals were met. Three patients were found to be ineligible after randomization, and one patient had a protocol violation. Therefore, 38 patients were included in the evaluable population (Figure 1). The majority of patients were male (73.7%), and the most common primary diagnosis was NMD (45%) (Table 1). Data regarding subject characteristics by study site and diagnosis are presented in Supplementary Tables S1–2. In the 24 patients with available data, AHI at enrollment was 30.9±26.3/h (range 5.3–105.8). NIV device settings were not significantly different between the two study nights (AutoEPAP and manual EPAP) (Table 2).
Figure 1.
CONSORT diagram for the study.
Table 1.
Demographics, clinical characteristics and non-invasive ventilation settings at baseline
| Patients (n=38) | |
|---|---|
| Gender, n (%) | |
| Male | 28 (73.7) |
| Female | 10 (26.3) |
| Age, years | |
| Mean ± SD (median) | 55.33 ± 16.01 (58.53) |
| Min, Max | 18.0, 81.0 |
| Race, n (%) | |
| Asian | 1 (2.6) |
| Black/African American | 8 (21.1) |
| Native Hawaiian or other Pacific Islander | 1 (2.6) |
| White | 23 (60.5) |
| Unknown/not available | 5 (13.2) |
| Body mass index, kg/m2 | |
| Mean ± SD (median) | 31.41 ± 9.84 (30.12) |
| Min, Max | 15.0, 57.2 |
| Primary diagnosis, n (%) | |
| Chronic obstructive pulmonary disease | 11 (28.9) |
| Neuromuscular disease | 17 (44.7) |
| Obesity hypoventilation syndrome | 4 (10.5) |
| Other | 6 (15.8) |
| Apnoea-hypopnoea index, events/hour | |
| Mean ± SD (median) | 30.91 ± 26.29 (17.80) |
| Min, Max | 5.3, 105.8 [24] |
| Ventilator dependent, n (%) | |
| No | 31 (81.6) |
| Yes | 7 (18.4) |
| Device mode at enrollment, n (%) | |
| Bilevel ST | 12 (31.6) |
| VAPS | 26 (68.4) |
| Device settings at enrollment | |
| IPAP, cmH2O: | |
| Mean ± SD (median) | 16.8 ± 4.7 (17.0) |
| Min, Max [n] | 6, 29 [29] |
| EPAP, cmH2O: | |
| Mean ± SD (median) | 7.4 ± 3.4 (6.0) |
| Min, Max [n] | 4, 16 [37] |
| Back-up rate, breaths per min: | |
| Mean ± SD (median) | 8.74 ± 6.22 (12.00) |
| Min, Max [n] | 0.0, 18.0 [38] |
| Duration of NIV treatment, years | |
| Mean ± SD (median) | 3.16 ± 3.75 (1.66) |
| Min, Max | 0.2, 17.0 |
SD, standard deviation; VAPS, volume-assured pressure support.
Table 2.
Non-invasive ventilation device settings and reported pressures during study nights
| Mean±SD (Median) Min,Max [N] | |||
|---|---|---|---|
| iVAPS Settings | iVAPS Auto EPAP (N = 38) | iVAPS Manual EPAP (N = 38) | p-value |
| EPAP Setting (cmH2O) | N/A | 7.6 ± 3.5 (6.5) | N/A |
| 4, 16 [38] | |||
| Min EPAP Setting (cmH2O) | 6.3 ± 2.5 (5.0) | N/A | N/A |
| 4, 15 [37] | |||
| Max EPAP Setting (cmH2O) | 15.0 ± 1.3 (15.0) | N/A | N/A |
| 9, 20 [37] | |||
| Min PS (cmH2O) | 3.2 ± 1.2 (4.0) | 3.1 ± 1.2 (3.0) | 0.57 |
| 2, 6 [37] | 2, 6 [38] | ||
| Max PS (cmH2O) | 19.4 ± 2.0 (20.0) | 19.3 ± 2.2 (20.0) | 0.53 |
| 10, 20 [37] | 10, 22 [38] | ||
| Target Va (L/min) | 6.03 ± 2.31 (5.20) | 6.01 ± 2.32 (5.20) | 0.32 |
| 3.6, 16.7 [37] | 3.5, 16.7 [38] | ||
| iVAPS Report Parameters | |||
| EPAP Median (cmH2O) | 10.24 ± 3.02 (10.50) | 7.04 ± 3.28 (5.80) | 0.0002 |
| 4.2, 14.8 [36] | 3.8, 15.6 [37] | ||
| EPAP 95% (cmH2O) | 13.30 ± 2.30 (14.20) | 7.42 ± 3.29 (6.40) | <0.0001 |
| 6.4, 16.0 [36] | 4.0, 16.0 [37] | ||
| IPAP Median (cmH2O) | 19.82 ± 6.11 (18.00) | 17.43 ± 5.26 (16.60) | 0.0003 |
| 11.0, 34.8 [36] | 7.8, 28.0 [37] | ||
| IPAP 95% (cmH2O) | 25.93 ± 6.55 (24.00) | 23.06 ± 5.06 (23.80) | 0.0001 |
| 16.2, 35.6 [36] | 12.4, 32.0 [37] | ||
| Vt Median (L) | 0.486 ± 0.131 (0.456) | 0.460 ± 0.131 (0.432) | 0.06 |
| 0.35, 1.00 [36] | 0.27, 1.00 [37] | ||
| Vt 95% (L) | 0.744 ± 0.160 (0.744) | 0.698 ± 0.198 (0.702) | 0.18 |
| 0.43, 1.00 [36] | 0.11, 1.00 [37] | ||
| RR Median (BPM) | 16.8 ± 3.3 (16.0) | 17.3 ± 3.7 (17.0) | 0.35 |
| 12, 26 [36] | 12, 27 [37] | ||
| RR 95% (BPM) | 21.9 ± 4.5 (21.0) | 21.5 ± 5.3 (20.0) | 0.78 |
| 15, 32 [36] | 14, 38 [37] | ||
| RSBI | 37.02 ± 12.27 (36.05) | 40.94 ± 16.04 (36.82) | 0.13 |
| 16.0, 66.7 [36] | 16.0, 81.5 [37] | ||
Values are mean ± standard deviation (median), with Min, Max values [n] below.
EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure; iVAPS, intelligent volume-assured pressure support; N/A, not applicable; PS, pressure support; Va, alveolar ventilation.
P-values generated from a paired t-test or sign test, as appropriate.
Primary endpoint
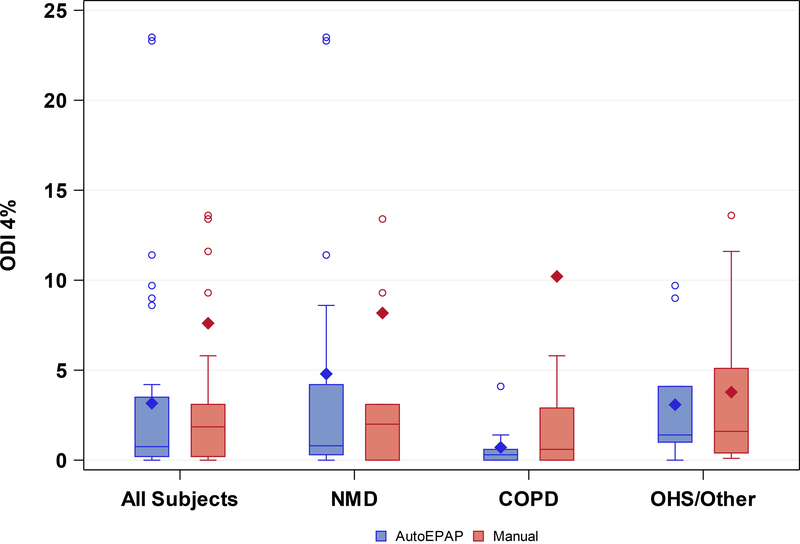
Mean ODI4% was lower during NIV with AutoEPAP versus manual EPAP (Table 3 and Figure 2).Treatment order had no effect on the results (p=0.35), and data were homogeneous across study sites (Kruskal-Wallis p-value <0.15). AutoEPAP was statistically non-inferior using the proposed one-sided paired t-test using a significance level of 0.05 and a non-inferiority margin of 2 events/hour (p=0.01). However, testing showed that the data had a non-normal distribution (Shapiro-Wilk test, p<0.0001). A non-parametric test (Wilcoxon signed rank) highlighted the non-inferiority of AutoEPAP compared with manual EPAP (p<0.0001) (Table 3). The mean difference in ODI4% between AutoEPAP and manual EPAP was not significantly different between subgroups based on disease type (i.e. neuromuscular, COPD, or OHS and other combined) (Figure 2) (p=0.49) or ventilator dependency status (p=0.34).
Table 3.
Oxygen desaturation index 4% during non-invasive ventilation with automatic versus manual expiratory positive airway pressure (n=38)
| AutoEPAP | Manual EPAP | Paired Difference | 95% upper one-sided confidence bound | p-value | |
|---|---|---|---|---|---|
| ODI4%, /h | 3.16 ± 5.67 | 7.61 ± 18.87 | −4.45 ± 17.23 (−96.7, 10.1) | 0.27 | 0.01341 |
| <0.00012 |
Values are mean ± standard deviation (range), unless otherwise stated.
EPAP, expiratory positive airway pressure; ODI4%, oxygen desaturation index 4%.
One-sided paired t-test
Wilcoxon signed-rank test.
Figure 2.
Difference in ODI4% between AutoEPAP and manual EPAP in all subjects, and subjects grouped by etiology of respiratory failure. OHS and other binned together due to small sample size. ODI4% while using AutoEPAP was non-inferior to manual EPAP in all subjects. There were no significant differences in ODI4% across the subgroups of neuromuscular disease, COPD, and OHS and other combined.
Note: Three outliers not displayed where ODI 4% > 40
Secondary endpoints
Settings for iVAPS mode and resulting device output on the two PSG study nights are shown in Table 2. Mean EPAP and IPAP were higher during AutoEPAP, but ventilation and mean rapid shallow breathing index (a marker of work of breathing) were similar on both nights. Mean oxygen saturation and transcutaneous carbon dioxide were similar with the two NIV modes (Table 4). On average, patients spent significantly less time in Stage N1 sleep, more time in REM sleep, and had significantly fewer arousals during AutoEPAP versus manual EPAP (Table 4). Data for subgroups of neuromuscular, COPD, and OHS or Other subjects are reported in Supplementary Tables S3–5. Data for ventilator dependent and non-dependent subgroups are reported in the Supplementary Tables S6–7.
Table 4.
Sleep parameters
| Mean±SD (Median) (Min-Max)[N] | |||
|---|---|---|---|
| iVAPS Auto EPAP (N = 38) | iVAPS Manual EPAP (N = 38) | p-value | |
| Sleep parameters | |||
| TST, min | 303.3 ± 79.58 (315.8) | 308.0 ± 82.37 (328.3) | 0.75 |
| 55.9, 410.5 [38] | 47.5, 450.5 [38] | ||
| Stage N1, % of TST | 16.18 ± 11.50 (12.90) | 20.47 ± 14.69 (17.10) | 0.01 |
| 1.9, 54.6 [38] | 1.4, 73.1 [38] | ||
| Stage N2, % of TST | 50.03 ± 13.83 (51.35) | 48.52 ± 17.20 (48.65) | 0.55 |
| 11.9, 72.9 [38] | 6.5, 76.6 [38] | ||
| Stage N3, % of TST | 17.93 ± 17.98 (11.30) | 17.74 ± 18.36 (14.45) | 0.61 |
| 0.0, 77.3 [38] | 0.0, 74.8 [38] | ||
| REM, % of TST | 15.58 ± 8.54 (16.05) | 12.76 ± 7.44 (12.80) | 0.04 |
| 0.0, 32.1 [38] | 0.0, 26.3 [38] | ||
| Arousals, /h | 25.85 ± 17.39 (20.70) | 31.91 ± 20.19 (24.70) | 0.04 |
| 2.9, 87.0 [38] | 3.6, 87.2 [38] | ||
| Sleep efficiency, % | 73.70 ± 16.41 (73.95) | 73.89 ± 19.05 (76.47) | 0.95 |
| 25.6, 94.1 [38] | 11.1, 98.1 [38] | ||
| Device Data | |||
| AHIflow, /h | 4.71 ± 5.55 (2.10) | 7.84 ± 15.39 (3.80) | 0.22 |
| 0.2, 21.4 [36] | 0.0, 91.2 [37] | ||
| Mean SpO2, % | 94.24 ± 2.36 (94.00) | 94.53 ± 2.78 (95.00) | 0.81 |
| 88.0, 99.0 [34] | 88.0, 99.0 [36] | ||
| Median SpO2, % | 94.53 ± 2.36 (95.00) | 94.86 ± 2.66 (95.00) | 0.69 |
| 89.0, 99.0 [34] | 89.0, 99.0 [36] | ||
| Mean PCO2, mmHg | 45.49 ± 6.62 (47.00) | 45.38 ± 7.17 (44.80) | 0.83 |
| 33.2, 62.7 [35] | 33.2, 65.2 [36] | ||
| Median PCO2, mmHg | 45.67 ± 6.62 (48.10) | 45.27 ± 7.13 (44.75) | 0.44 |
| 33.2, 63.0 [35] | 32.9, 65.7 [36] | ||
Values are mean ± standard deviation (median), with Min, Max values [n] below.
AHIflow, apnoea-hypopnoea index based on device flow signal; pCO2, carbon dioxide pressure; REM, rapid eye movement sleep; SpO2, oxygen saturation; TST, total sleep time.
P-values generated from a paired t-test or sign test, as appropriate.
Adverse events
No serious adverse events were reported during the study. One non-serious adverse event (asthma exacerbation) occurred during the second PSG night (manual EPAP) in one patient, which resolved after utilization of routine treatment. This was determined by the site investigator to be not related to the device or device mode.
Discussion
In the therapy of obstructive sleep apnoea, automatic EPAP algorithms have long been shown to be effective in assessing and treating upper airway obstruction. Many patients have complicated issues with sleep-disordered breathing, because they have both chronic hypoventilation as well as abnormalities in upper airway collapsibility. Since both factors need to be addressed, the goal of this investigation was to evaluate the ability of an automatic EPAP algorithm in a VAPS mode to control upper airway obstruction while remaining effective at treating hypoventilation, without requiring titration by a sleep laboratory technician. The results of this study showed that AutoEPAP was non-inferior to manual EPAP for improving ODI4%, as a measure of upper airway obstruction, in patients with respiratory failure secondary to a variety of underlying hypoventilation disorders. The mean difference between AutoEPAP and manual EPAP in normalizing ODI4% was similar across disease types and ventilator dependency.
AutoEPAP resulted in higher delivered mean EPAP levels than those set under manual EPAP. While higher EPAP might improve upper airway resistance and potentially facilitate triggering, one concern of might be a negative effect on ventilation, work of breathing, or sleep quality. However, we did not observe any statistically significant difference in modes with respect to ventilation parameters, or gas exchange, suggesting that AutoEPAP did not impair respiratory mechanics, and pressure support was appropriately maintained by the iVAPS algorithm. In addition, parameters reflecting sleep quality were improved, including decreased arousals and increased REM sleep.
We utilized ODI4% as the primary outcome rather than AHI, as AHI determined by the device would not meet current standards for classifying hypopnoeas, and would be utilizing the same algorithms being used to auto titrate the EPAP level. Moreover, transient hypoventilation may be seen in this population, which may be mistaken for upper airway obstructive events when relying solely on flow signals. The effectiveness of AutoEPAP on ODI4% without worsening ventilation suggests the algorithm is able to classify appropriately events as obstructive; if events were due to hypoventilation they might have persisted or even worsened at higher EPAP levels. The ODI4% findings were similar to those observed in the device-measured residual respiratory event index, which were similar between AutoEPAP and manual titration.
The results of our study show a similar outcome to a recent investigation of AutoEPAP in patients with both chronic hypoventilation and upper airway obstruction 16. However, there were some differences between the studies. We evaluated both ventilation dependent and non-dependent patients using a home ventilator, versus non-dependent patients using a bilevel device in the previous study. In addition, NMD was predominant in our study, whereas OHS was most common in the prior study. The studies also had different primary endpoints – ODI4% in our case and AHI in the previous study. Given the differences between the studies in terms of primary outcome measures and underlying diseases, their combined results provide growing evidence of the efficacy of AutoEPAP.
Auto-titrating NIV may have benefits with respect to sleep quality. A prior study of auto-titrating pressure support reported less percent stage N1 sleep and fewer arousals compared to standard NIV 13. Similarly, patients in our study spent significantly less time in stage N1 sleep with AutoEPAP versus manual EPAP, and the mean number of arousals was significantly lower. This finding could indicate easier sleep initiation and a trend towards a longer period of deep sleep during use of automatic versus manual settings 13. Patients receiving home-based NIV have reported more restful sleep when using iVAPS compared with conventional high-intensity NIV, although objective sleep quality parameters were not significantly different 6. It is possible that trends towards improved sleep during iVAPS could be enhanced when iVAPS is used with Auto- versus manual EPAP, but this remains to be determined in future studies.
Adherence with therapy is another important factor to consider during long-term use of NIV. In one study, iVAPS led to one additional hour per night of use compared with standard pressure support 14. Such improvements in adherence could have an impact on patient outcomes 18, and therefore future studies are warranted as to whether the addition of AutoEPAP further improves use.
Strengths of this study include its randomized design, multicentre performance, centralized and blinded scoring of sleep studies, experienced clinicians/investigators, and the inclusion of a heterogeneous population reflective of a clinical population. However, there were a number of limitations. The two NIV modes were only compared over a single night of therapy. Therefore, comparative longer term effects of the Auto-EPAP mode need to be investigated, including effectiveness and compliance particularly given the higher EPAP pressures observed with AutoEPAP. We studied patients already using NIV, in whom previous EPAP settings were available. While this primarily had the effect of minimizing the need for manual titration, we cannot generalize our findings to an NIV-naïve group. We did not collect detailed lung function or blood gas information, and instead relied on the referring clinician diagnosis and patient history. Most patients had been on NIV for years and collecting contemporary data would have been difficult, but nonetheless may have provided additional information. The study cohort had few women, who may benefit from different AutoEPAP algorithms from men 19. Finally, we cannot draw specific non-inferiority conclusions for each group, as our numbers in each group were relatively small, and this would require a dedicated study for each group rather than our approach of showing overall non-inferiority.
In conclusion, a single night of NIV using iVAPS with AutoEPAP was non-inferior to iVAPS with manual EPAP with respect to mean ODI4% in a group of patients with respiratory failure secondary to NMD, COPD, OHS and other causes. There was no suggestion of compromise in ventilation, and sleep parameters improved while using AutoEPAP. Additional research is needed to compare these modes over longer treatment durations and to evaluate the use of AutoEPAP iVAPS in NIV-naïve patients.
Supplementary Material
Summary at a Glance.
Automated EPAP titration may be helpful in treating patients with respiratory failure and upper airway obstruction. This study demonstrates that for a single night titration, an automated EPAP algorithm is as effective as manually-set EPAP for treating desaturation, without compromising ventilation and potentially improving sleep quality.
Acknowledgements
The authors thank Pamela Deyoung, RPSGT for study coordination and scoring of sleep studies, and Colleen Kelly, PhD, for assistance with the analysis.
Disclosure statement
ResMed Corp., San Diego, CA, USA provided funding for the data collection platform and data analysis of this study. These funds were provided to the authors institutions but not to the authors directly. The use of the funds were overseen by each institutions office of research/special contracts. LFW led the design of the study protocol with support from AVB and MEC; study site investigators had full oversight of the study conduct. Adam V. Benjafield, and Maureen E. Crocker are employed by ResMed; however we do not expect the company to gain or lose financially as a result of this study. Statistical analyses were completed by an independent statistician, paid for by ResMed Corp. Medical editing support was provided by Nicola Ryan, BSc, an independent medical writer, funded by ResMed.
Dr. Orr has received an unrestricted grant from the American Thoracic Society Foundation partnered with ResMed. Drs. Coleman, Criner, Tsai and Sundar have no conflicts of interest to disclose. Dr. Benjafield and Ms. Crocker are employees of ResMed. Ms. Willes is an independent statistician who was funded by ResMed. Dr. Malhotra is the principal investigator on NIH RO1 HL085188, K24 HL132105, T32 HL134632 and co-investigator on R21 HL121794, R21 HL138075, RO1 HL 119201, RO1 HL081823, UG1 HL139117-01, CPLGO (Center for Physiological Genomics of Low Oxygen), RO1 CA215405, RO1 HL133847, and mentor on K23 HL133489, F32 HL136202 and F32 HL131306. Dr. Owens has received travel reimbursement and honorarium from ResMed and Itamar Medical and is a consultant for Novartis. ResMed, Inc. provided a philanthropic donation to the UC San Diego in support of a sleep center. Dr. Wolfe has received grant funding from ResMed, Hill-Rom and Synapse Biomedical. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Data availability statement
Data from this study will not be shared to the public. Additional study information can be requested from the corresponding author.
References
- 1.Nicolini A, Banfi P, Grecchi B, Lax A, Walterspacher S, Barlascini C, Robert D. Non-invasive ventilation in the treatment of sleep-related breathing disorders: A review and update. Rev Port Pneumol. 2014; 20: 324–35. [DOI] [PubMed] [Google Scholar]
- 2.Fanfulla F, Delmastro M, Berardinelli A, Lupo NDA, Nava S. Effects of Different Ventilator Settings on Sleep and Inspiratory Effort in Patients with Neuromuscular Disease. American journal of respiratory and critical care medicine. 2005; 172: 619–24. [DOI] [PubMed] [Google Scholar]
- 3.Chiu KL, Ryan CM, Shiota S, Ruttanaumpawan P, Arzt M, Haight JS, Chan CT, Floras JS, Bradley TD. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006; 174: 1378–83. [DOI] [PubMed] [Google Scholar]
- 4.Steier J, Jolley CJ, Seymour J, Kaul S, Luo YM, Rafferty GF, Hart N, Polkey MI, Moxham J. Sleep-disordered breathing in unilateral diaphragm paralysis or severe weakness. The European respiratory journal. 2008; 32: 1479–87. [DOI] [PubMed] [Google Scholar]
- 5.Steier J, Jolley CJ, Seymour J, Roughton M, Polkey MI, Moxham J. Neural respiratory drive in obesity. Thorax. 2009; 64: 719–25. [DOI] [PubMed] [Google Scholar]
- 6.Ekkernkamp E, Storre JH, Windisch W, Dreher M. Impact of intelligent volume-assured pressure support on sleep quality in stable hypercapnic chronic obstructive pulmonary disease patients: a randomized, crossover study. Respiration. 2014; 88: 270–6. [DOI] [PubMed] [Google Scholar]
- 7.Murphy PB, Davidson C, Hind MD, Simonds A, Williams AJ, Hopkinson NS, Moxham J, Polkey M, Hart N. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax. 2012; 67: 727–34. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson TT, Smith SB, Siddique T, Sufit R, Ajroud-Driss S, Coleman JM 3rd, Wolfe LF. Respiratory Pattern and Tidal Volumes Differ for Pressure Support and Volume-assured Pressure Support in Amyotrophic Lateral Sclerosis. Annals of the American Thoracic Society. 2017; 14: 1139–46. [DOI] [PubMed] [Google Scholar]
- 9.Nilius G, Katamadze N, Domanski U, Schroeder M, Franke KJ. Non-invasive ventilation with intelligent volume-assured pressure support versus pressure-controlled ventilation: effects on the respiratory event rate and sleep quality in COPD with chronic hypercapnia. International journal of chronic obstructive pulmonary disease. 2017; 12: 1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oscroft NS, Chadwick R, Davies MG, Quinnell TG, Smith IE. Volume assured versus pressure preset non-invasive ventilation for compensated ventilatory failure in COPD. Respir Med. 2014; 108: 1508–15. [DOI] [PubMed] [Google Scholar]
- 11.Pluym M, Kabir AW, Gohar A. The use of volume-assured pressure support noninvasive ventilation in acute and chronic respiratory failure: a practical guide and literature review. Hospital practice (1995). 2015; 43: 299–307. [DOI] [PubMed] [Google Scholar]
- 12.Battisti A, Tassaux D, Bassin D, Jolliet P. Automatic adjustment of noninvasive pressure support with a bilevel home ventilator in patients with acute respiratory failure: a feasibility study. Intensive Care Med. 2007; 33: 632–8. [DOI] [PubMed] [Google Scholar]
- 13.Jaye J, Chatwin M, Dayer M, Morrell MJ, Simonds AK. Autotitrating versus standard noninvasive ventilation: a randomised crossover trial. The European respiratory journal. 2009; 33: 566–71. [DOI] [PubMed] [Google Scholar]
- 14.Kelly JL, Jaye J, Pickersgill RE, Chatwin M, Morrell MJ, Simonds AK. Randomized trial of ‘intelligent’ autotitrating ventilation versus standard pressure support non-invasive ventilation: impact on adherence and physiological outcomes. Respirology. 2014; 19: 596–603. [DOI] [PubMed] [Google Scholar]
- 15.Hussein K Non invasive spontaneous dual ventilation in critically ill patients with chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2016; 65: 99–104. [Google Scholar]
- 16.McArdle N, Rea C, King S, Maddison K, Ramanan D, Ketheeswaran S, Erikli L, Baker V, Armitstead A, Richards G, Singh B, Hillman D, Eastwood P. Treating chronic hypoventilation with automatic adjustable versus fixed EPAP intelligent volume-assured positive airway pressure support (iVAPS): a randomized controlled trial. Sleep. 2017; 40: doi: 10.1093/sleep/zsx136. [DOI] [PubMed] [Google Scholar]
- 17.Berry RB, Chediak A, Brown LK, Finder J, Gozal D, Iber C, Kushida CA, Morgenthaler T, Rowley JA, Davidson-Ward SL. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2010; 6: 491–509. [PMC free article] [PubMed] [Google Scholar]
- 18.Borel JC, Pepin JL, Pison C, Vesin A, Gonzalez-Bermejo J, Court-Fortune I, Timsit JF. Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients. Respirology. 2014; 19: 857–65. [DOI] [PubMed] [Google Scholar]
- 19.McArdle N, King S, Shepherd K, Baker V, Ramanan D, Ketheeswaran S, Bateman P, Wimms A, Armitstead J, Richards G, Hillman D, Eastwood P. Study of a Novel APAP Algorithm for the Treatment of Obstructive Sleep Apnea in Women. Sleep. 2015; 38: 1775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.