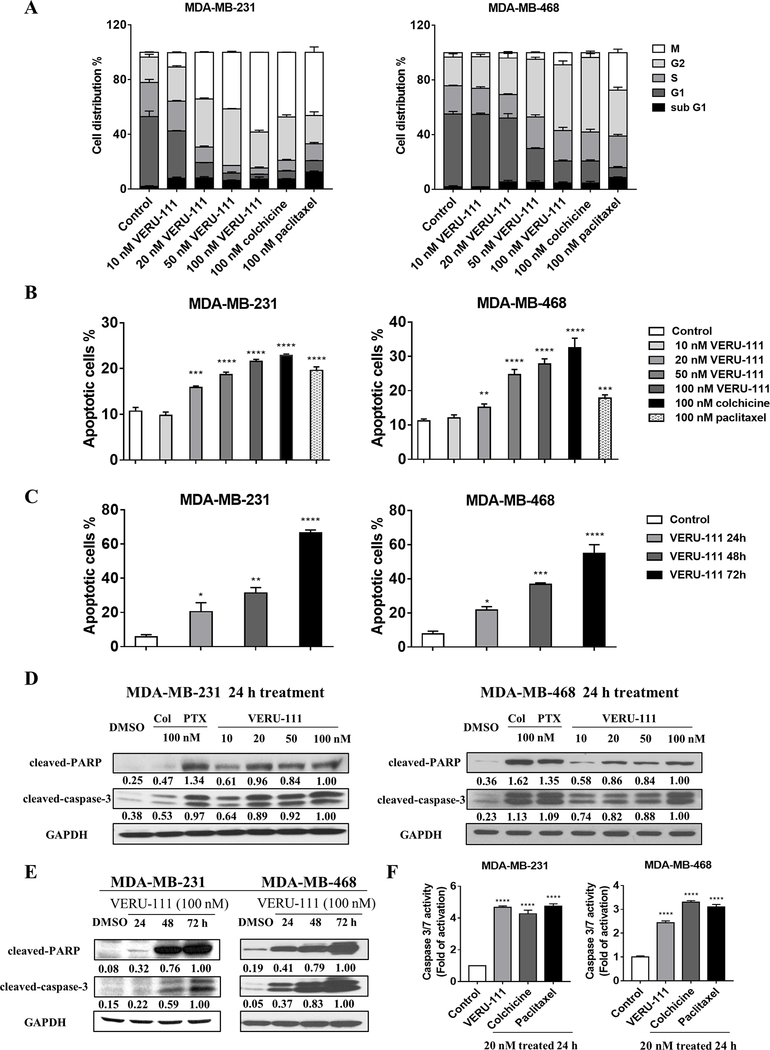
Figure 3.
VERU-111 induces G2/M cell cycle arrest and apoptosis in TNBC cells. (A) Cell cycle distribution was determined by flow cytometry after staining of MDA-MB-231 cells (left panel) and MDA-MB-468 cells (right panel) with phospho-Histone H3 (Ser10) and propidium iodide post-treatment with 100 nM colchicine or 100 nM paclitaxel as controls, or VERU-111 at concentrations of 10, 20, 50 and 100 nM for 24 h. The percentage of cells in each phase of the cell cycle is shown as the grand mean ± SEM calculated from three independent experiments. (B) Apoptosis was compared after treatment with the same drugs by Annexin-V/PI co-staining and flow cytometry analysis expressed as the grand mean of the apoptotic cells (%) ± SEM calculated from three independent experiments, as compared to Control. (C) Flow cytometry analysis of apoptotic cells detected by Annexin-V/PI staining in MDA-MB-231 (left panel) and MDA-MB-468 cells (right panel) treated with vehicle, or VERU-111 at 100 nM for 24, 48, or 72 h, expressed as the grand mean of the apoptotic cells (%)± SEM of three independent experiments, as compared to Control. (D) Cleaved-caspase-3 and PARP cleavage were determined by western blotting after treatments as in A. GAPDH was used as a loading control. Signal intensity was evaluated by ImageJ densitometry, with 100 nM VERU-111 treatment set to 1.00. (E) Cleaved-caspase-3 and PARP cleavage by western blotting following VERU-111 treatment (100 nM) for 24, 48 and 72 h. Signal intensity was evaluated by ImageJ densitometry, with 100 nM VERU-111 treatment at 72h set to 1.00. (F) Caspase 3/7 activity following treatment with 20 nM of each drug for 24h in MDA-MB-231 (left panel) and MDA-MB-468 (right panel) cells. Bar graphs represent the grand mean of the fold change of caspase 3/7 activity± SEM of three independent experiments as compared to Control.