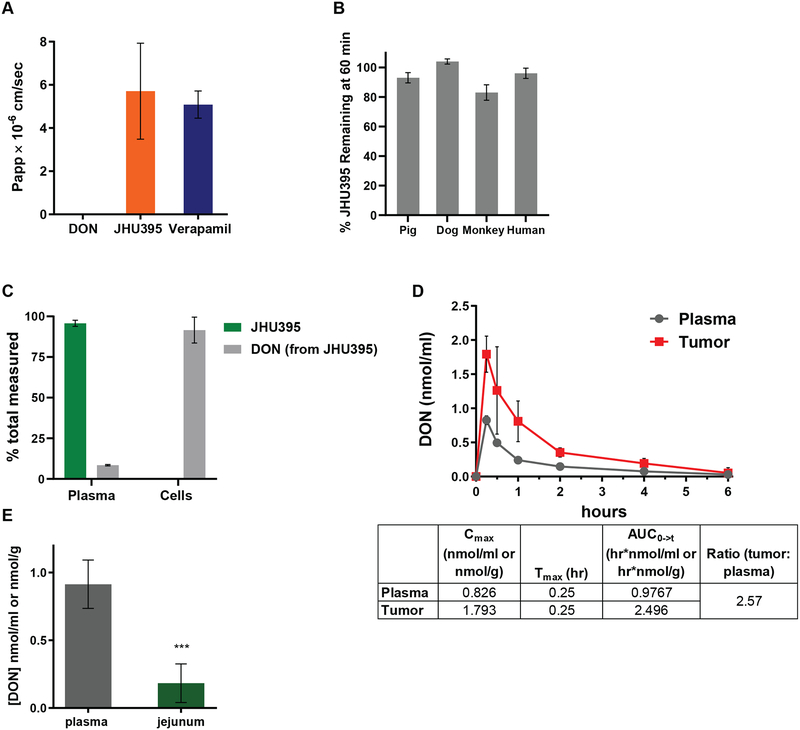
Figure 2:
JHU395 is a lipophilic, plasma stable prodrug GA that delivers DON to MPNST in vitro and in vivo. A) Calculated apparent permeability (Papp) of DON, JHU395, or Verapamil (positive control) following LC-MS quantification of donor and acceptor samples in a 5 hour parallel artificial membrane permeation assay. Data is +/− S.D. B) Percentage of quantified intact JHU395 in plasma from multiple species following 60 minutes incubation at 37°C. Data is +/− S.D. C) Percentage of quantified intact JHU395 and DON in human plasma and MPNST cells (sNF96.2) following one hour incubation with 20 μM JHU395. Data is +/− S.E.M. D) DON quantification (nmol/g tissue or nmol/ml plasma) following oral administration of 1.2 mg/kg JHU395 to flank MPNST B6 mice. Chart indicates DON Cmax, Tmax, and area under the curve (AUC) in tumor and plasma. Error bars are +/− S.D. E) DON quantification (nmol/g tissue or nmol/ml plasma) in plasma and jejunum thirty minutes after 1.2 mg/kg JHU395 to B6 mice. Error bars are +/− S.D. *** 0.0001 < p ≤ 0.001 by Student’s t-test.