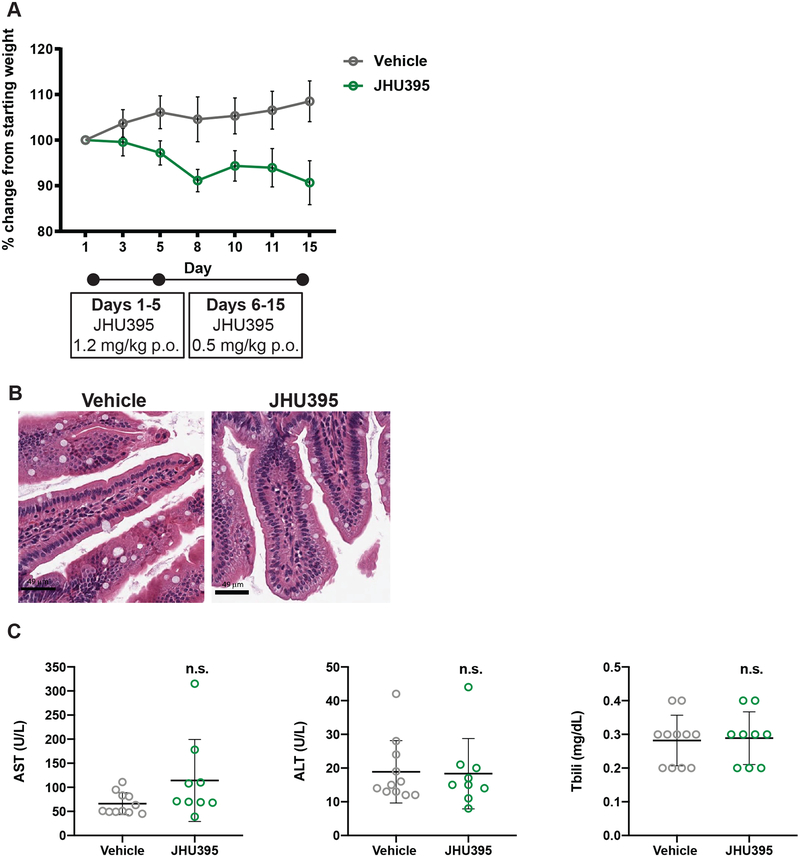
Figure 4:
Orally administered JHU395 has minimal GI or hepatotoxicity. A) Average weight change in flank MPNST mice treated during efficacy study with vehicle or JHU395 (1.2 mg/kg/day p.o. × 5 days, then 0.5 mg/kg p.o. × 9 days; n=9). Change from mean starting weight did not exceed 10%. B) Jejunal histology in a subset of vehicle and JHU395 treated mice after 14 days oral dosing. No increase in apoptotic figures or disruption of crypt architecture was observed. Scalebar = 49 μm. C) Markers of liver toxicity from blood (AST, ALT, total bilirubin) were measured in vehicle or JHU395 treated animals at sacrifice (day 15). All data shown is mean +/− S.D. p > 0.05 for each lab value by t-test.